Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus aureus
Abstract
:1. Introduction
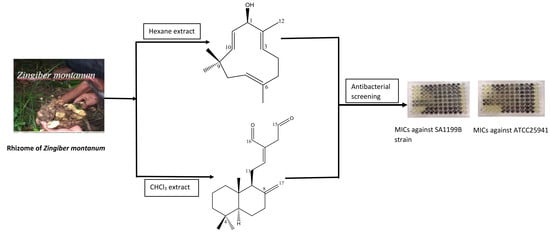
2. Results and Discussion
3. Material and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation of Compounds
3.4. Antibacterial Assay against Clinical Isolates of Multi-Drug Resistant and Methicillin Resistant Staphylococcus Aureus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 15 February 2018).
- World Health Organization (WHO). Antibiotic Resistance. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 18 November 2018).
- Faridi, A.; Kareshk, A.T.; Fatahi-Bafghi, M.; Ziasistani, M.; Ghahraman, M.R.K.; Seyyed-Yousefi, S.Z.; Shakeri, N.; Kalantar-Neyestanaki, D. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in clinical samples of patients with external ocular infection. Iran. J. Microbiol. 2018, 10, 215–219. [Google Scholar] [PubMed]
- Hollman, A. Digoxin comes from Digitalis lanata. Br. Med. J. 1996, 312, 912. [Google Scholar] [CrossRef]
- Alam, M.M.; Naeem, M.; Khan, M.M.A.; Uddin, M. Vincristine and Vinblastine Anticancer Catharanthus Alkaloids: Pharmacological Applications and Strategies for Yield Improvement. In Catharanthus Roseus; Springer: Cham, Switzerland, 2017; pp. 277–307. [Google Scholar]
- Patrick, G. The Opioid Analgesic. In An Introduction to Medicinal Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2013; p. 632. [Google Scholar]
- Butler, A.; Hensman, T. Drugs for the fever. Educ. Chem. 2000, 37, 151. [Google Scholar]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Saklani, A.; Jachak, S.M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007, 110, 200–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, P.; Zhang, G.; Xu, L.; Wang, D.; Wang, L.; Zeng, X.; Wang, Y. Design, synthesis and antibacterial activity of novel andrographolide derivatives. Bioorg. Med. Chem. 2010, 18, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial activity of hyperforin from St John’s wort against multi-resistant Staphylococcus aureus and Gram-positive bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef]
- Shiu, W.K.P.; Rahman, M.M.; Curry, J.; Stapleton, P.D.; Zloh, M.; Malkinson, J.P.; Gibbons, S. Antibacterial acylphloroglucinols from Hypericum olympicum. J. Nat. Prod. 2012, 75, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: Berlin/Heidelberg, Germany, 2007; p. 733. [Google Scholar]
- Farnsworth, N.R.; Bunyapraphatsara, N. Thai Medicinal Plants: Recommended for Primary Health Care System; Medicinal Plants Information Center: Bangkok, Thailand, 1992. [Google Scholar]
- Singh, C.B.; Manglembi, N.; Swapana, N.; Chanu, S.B. Ethnobotany, phytochemistry and pharmacology of Zingiber cassumunar Roxb. (Zingiberaceae). J. Pharmacogn. Phytochem. 2015, 4, 1–6. [Google Scholar]
- Sharma, G.J.; Thokchom, D.S. Antioxidant and radioprotective properties of Zingiber montanum (J. König) A. Dietr. Planta Med. 2011, 77, 127. [Google Scholar] [CrossRef]
- Thokchom, D.S.; Sharma, T.D.; Sharma, G.J. Radioprotective effect of rhizome extract of Zingiber montanum in Rattus norvegicus. Radiat. Environ. Biophys. 2012, 51, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Sultana, G.N.; Hossain, C.F. Antiulcer principle from Zingiber montanum. J. Ethnopharmacol. 2012, 141, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Jitoe, A.; Mabry, M.J. Isolation and structure determination of cassumunarins A, B and C: New anti-inflammatory antioxidants from a tropical ginger, Zingiber cassumunar. J. Am. Oil Chem. Soc. 1995, 72, 1053–1057. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Verma, S.K.; Iqbal, H.; Chanda, D.; Verma, R.K.; Darokar, M.P.; et al. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J. Sci. Food Agric. 2018, 98, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sy, K.L.; Brown, D.G. Labdane diterpenoids from Alpinia chinensis. J. Nat. Prod. 1997, 60, 904–908. [Google Scholar] [CrossRef]
- Firman, K.; Kinoshita, T.; Itai, A.; Sankawa, U. Terpenoids from Curcuma Heyneana. Phytochemistry 1988, 27, 3887–3891. [Google Scholar] [CrossRef]
- Takashi, K.; Nagao, R.; Masuda, T.; Hill, R.K.; Morita, M.; Takatani, M.; Sawada, S.; Okamoto, T. The chemistry of Zerumbone IV: Asymmetric synthesis of Zerumbol. J. Mol. Catal. B Enzym. 2002, 17, 75–79. [Google Scholar]
- Nathaniel, C.; Elaine-Lee, Y.L.; Yee, B.C.; How, C.W.; Yim, H.S.; Rasadee, A.; Ng, H.S. Zerumbone-loaded nanostructured lipid carrier induces apoptosis in human colorectal adenocarcinoma (Caco-2) cell line. Nanosci. Nanotechnol. Lett. 2016, 8, 294–302. [Google Scholar] [CrossRef]
- Cai, Z.; Yongpruksa, N.; Harmata, M. Total synthesis of the terpenoid buddledone A: 11-membered ring-closing metathesis. Org. Lett. 2012, 14, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Simova, S.D.; Bozhkova, N.V.; Orahovats, A.S. 1H and 13C NMR studies of some germacrones and isogermacrones. Org. Magn. Reson. 1984, 22, 431–433. [Google Scholar] [CrossRef]
- Brieskorn, C.H.; Noble, P. Furanosesquiterpenes from the essential oil of myrrh. Phytochemistry 1983, 22, 1207–1211. [Google Scholar] [CrossRef]
- Uchio, Y. Constituents of the essential oil of Chrysanthemum japonense. Nojigiku alcohol and its acetate. Bull. Chem. Soc. Jpn. 1978, 51, 2342–2346. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Pinheiro, C.D.; Orlandi, P.P.; Pinheiro, C.C.; Sontes, G.S. Zerumbone from Zingiber zerumbet (L.) smith: A potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2018, 18, 301. [Google Scholar]
- Gibbons, S.; Udo, E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 2000, 14, 139–140. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; Ruble, C.A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993, 37, 1086–1094. [Google Scholar] [CrossRef]
- Ross, J.L.; Farrell, A.M.; Eady, E.A.; Cove, J.H.; Cunliffe, W.J.J. Characterisation and molecular cloning of the novel macrolide-streptogramin B resistance determinant from Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1989, 24, 851–862. [Google Scholar] [CrossRef]
- Richardson, J.F.; Reith, S. Characterization of a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J. Hosp. Infect. 1993, 25, 45–52. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |


| Extracts/Antibiotic | MICs in µg/mL | ||||
|---|---|---|---|---|---|
| SA1199B | XU212 | EMRSA15 | RN4229 | ATCC25941 | |
| n-Hexane | 128 | 128 | 256 | 256 | 128 |
| Chloroform | 64 | 128 | 128 | 128 | 256 |
| Methanol | >512 | >512 | >512 | >512 | >512 |
| Norfloxacin | 32 | 64 | 16 | 8 | 16 |
| Position | 1H | 13C | HMBC | |
|---|---|---|---|---|
| 2J | 3J | |||
| 1 | 1.06, m, 1H; 1.68, m, 1H | 39.4 | - | C-9 |
| 2 | 1.50, m, 1H; 1.57, m, 1H | 19.5 | C-3 | C-4 |
| 3 | 1.18, m, 1H; 1.41, m, 1H | 42 | - | C-18, C19 |
| 4 | - | 33.6 | - | - |
| 5 | 1.13, m, 1H | 55.8 | C6 | C3, C7, C9, C19, C20 |
| 6 | 1.34, m, 1H; 1.75, m, 1H | 24.4 | C5, C7 | C10 |
| 7 | 2.02, m, 1H; 2.42, m, 1H | 38.1 | C8 | C5, C17 |
| 8 | - | 148.4 | - | - |
| 9 | 1.90, m, 1H | 56.6 | C10, C11 | C12, C17, C20 |
| 10 | - | 39.8 | - | - |
| 11 | 2.31, m, 1H; 2.49, m, 1H | 24.8 | C9 | C8, C13 |
| 12 | 6.76, t, J = 6.6 Hz, 1H | 160.4 | - | C9, C14, C16 |
| 13 | - | 135 | - | - |
| 14 | 3.41, d, J = 16.8 Hz, 1H | 39.6 | C13 | C12, C16 |
| 3.46, d, J = 16.7 Hz, 1H | ||||
| 15 | 9.63, t, J = 14.4 Hz, 1H | 197.5 | C14 | C13 |
| 16 | 9.40, s, 1H | 193.8 | C13 | C12, C14 |
| 17 | 4.36, s, 1H; 4.86, s, 1H | 108.1 | C8 | C7, C9 |
| 18 | 0.88, s, 3H | 33.7 | - | C3, C5, C19 |
| 19 | 0.82, s, 3H | 22.1 | - | C3, C5, C18 |
| 20 | 0.72, s, 3H | 14.6 | C10 | C5, C9 |
| Position | 1H | 13C | HMBC | |
|---|---|---|---|---|
| 2J | 3J | |||
| 1 | 4.63, d, J = 7.5 Hz, 1H | 78.8 | - | C3, C10, C12 |
| 2 | - | 142.2 | - | - |
| 3 | 5.20, d, J = 7.5 Hz, 1H | 124.8 | - | C1, C12 |
| 4 | 2.20, m, 1H; 2.24, m, 1H | 24.4 | C3 | C6 |
| 5 | 2.35, m, 2H | 39.5 | - | C3, C13 |
| 6 | - | 133.2 | - | - |
| 7 | 4.82, dd, J = 10.2, 4.4 Hz, 1H | 125 | - | C5, C9 |
| 8 | 1.87, m, 1H; 2.32, m, 1H | 42.4 | C7 | C6, C10 |
| 9 | - | 37.3 | - | - |
| 10 | 5.23, d, J = 16.2 Hz, 1H | 139.5 | - | C1, C14, C15 |
| 11 | 5.56, dd, J = 16.2, 7.5 Hz, 1H | 131.2 | C1 | C9, C13 |
| 12 | 1.65, s, 3H | 12.8 | C2 | C1, C3 |
| 13 | 1.43, s, 3H | 15.3 | C6 | C5, C7 |
| 14 | 1.04, s, 3H | 24.9 | C9 | C8, C10, C15 |
| 15 | 1.06, s, 3H | 29.9 | C9 | C8, C10, C14 |
| Compound | SA1199B | XU212 | ATCC25941 | RN4220 | EMRSA15 | MRSA27819 | MRSA340702 |
|---|---|---|---|---|---|---|---|
| 1 | 0.212 | 0.424 | 0.212 | 0.212 | 0.212 | 0.424 | 0.424 |
| 2 | 0.291 | 0.582 | 0.582 | 0.582 | 0.145-0.291 | 0.582 | >0.582 |
| 3 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 |
| 4 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 | >0.582 |
| 5 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 | >0.587 |
| 6 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 | >0.557 |
| 7 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 | >0.831 |
| 8 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 | >0.842 |
| Norfloxacin | 0.100 | 0.200 | 0.050 | 0.025 | 0.050 | 0.100 | 0.401 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, H.; Pendry, B.; Rahman, M.M. Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus aureus. Molecules 2019, 24, 385. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030385
Siddique H, Pendry B, Rahman MM. Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus aureus. Molecules. 2019; 24(3):385. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030385
Chicago/Turabian StyleSiddique, Holly, Barbara Pendry, and M. Mukhlesur Rahman. 2019. "Terpenes from Zingiber montanum and Their Screening against Multi-Drug Resistant and Methicillin Resistant Staphylococcus aureus" Molecules 24, no. 3: 385. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030385