Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats
Abstract
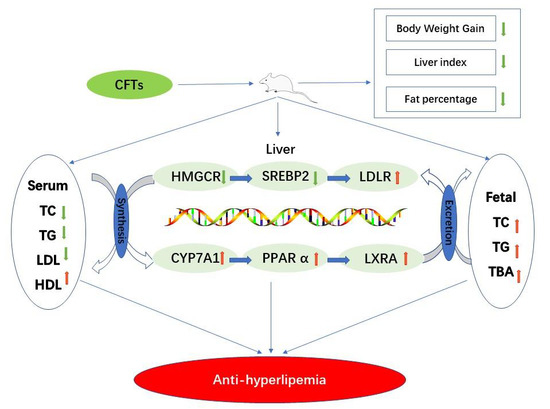
:1. Introduction
2. Results
2.1. Weight Gain, Food Intake, Fat Ratio, and Liver Index
2.2. Serum and Liver Lipid Levels in Rats
2.3. Serum MDA, SOD in Rats
2.4. TC, TG and TBA in Feces
2.5. Liver and Kidney Histology
2.6. Adipose Tissues Histology
2.7. Differential Gene Expression Analysis in Liver
2.8. CFTs Affects the Expressions of Lipid Metabolism Target Genes in Liver
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Diets
4.3. Serum Biochemical Analysis
4.4. Histological Staining
4.5. Gene Expression Tag Profiling
4.6. Quantitative RT-PCR
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Akiyama, T.; Tachibana, I.; Shirohara, H.; Watanabe, N.; Otsuki, M. High-Fat Hypercaloric Diet Induces Obesity, Glucose Intolerance and Hyperlipidemia in Normal Adult Male Wistar Rat. Diabetes Res. Clin. Pract. 1996, 31, 27–35. [Google Scholar] [CrossRef]
- Parhofer, K.G. The Treatment of Disorders of Lipid Metabolism. Deutsch. Ärzteblatt Int. 2016, 113, 261–268. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, J. The role of statins in clinical medicine–LDL–cholesterol lowering and beyond. Swiss Med. Wkly. 2006, 136, 41–49. [Google Scholar] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Ding, C.; Huang, L.; Bai, Y.; Xiao, T.; Guo, J.; Su, Z. The effects of COST on the differentiation of 3T3-L1 preadipocytes and the mechanism of action. Saudi J. Biol. Sci. 2017, 24, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.Y.; Guo, J.; Su, Z.Q. The preparation and the anti-obesity activity of chitosan oligosaccharide film coating tablets. Basic Clin. Pharmacol. Toxicol. 2016, 119, 29. [Google Scholar]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Z.; Guo, S.; Meng, X.; Chang, X. Polyphenol-rich extract from wild Lonicera caerulea berry reduces cholesterol accumulation by mediating the expression of hepatic miR-33 and miR-122, HMGCR, and CYP7A1 in rats. J. Funct. Foods 2018, 40, 648–658. [Google Scholar] [CrossRef]
- Jiao, R.; Zhang, Z.; Yu, H.; Huang, Y.; Chen, Z.Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010, 21, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.-J.; Lee, S.-J. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- Su, Z.; Guo, J.; Yang, Q. Chitosan Oligosaccharide Tablet and Preparation Method Thereof. Patent No. ZL201510988018.1, 24 December 2015. [Google Scholar]
- Lee, H.S.; Lee, Y.J.; Chung, Y.H.; Nam, Y.; Kim, S.T.; Park, E.S.; Hong, S.M.; Yang, Y.K.; Kim, H.C.; Jeong, J.H. Beneficial Effects of Red Yeast Rice on High-Fat Diet-Induced Obesity, Hyperlipidemia, and Fatty Liver in Mice. J. Med. Food 2015, 18, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, N.; Ghayour Mobarhan, M.; Sahebkar, A.; Pasdar, A. Molecular aspects of hypercholesterolemia treatment: Current perspectives and hopes. Ann. Med. 2018, 50, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, F.; Zhang, X.J.; Jin, R.M.; Li, X. Effect of high-fat diet on cholesterol metabolism in rats and its association with Na(+)/K(+)-ATPase/Src/pERK signaling pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Shi, F.; Li, J.; Yang, L.; Hou, G.; Ye, M. Hypolipidemic effect and protection ability of liver-kidney functions of melanin from Lachnum YM226 in high-fat diet fed mice. Food Funct. 2018, 9, 880–889. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Public Policy Committee of the American Association for the Study of Liver. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef]
- Cao, P.; Huang, G.; Yang, Q.; Guo, J.; Su, Z. The effect of chitooligosaccharides on oleic acid-induced lipid accumulation in HepG2 cells. Saudi Pharm. J. 2016, 24, 292–298. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siow, H.-L.; Choi, S.-B.; Gan, C.-Y. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J. Funct. Foods 2016, 27, 600–611. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Takabe, K.; Dozmorov, M.; Nagahashi, M.; Zhou, H. Bile acids as global regulators of hepatic nutrient metabolism. Liver Res. 2017, 1, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; Chen, J.; Jiao, R.; Peng, C.; Zuo, Y.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Huang, Y.; et al. Cholesterol-Lowering Activity of Sesamin Is Associated with Down-Regulation on Genes of Sterol Transporters Involved in Cholesterol Absorption. J. Agric. Food Chem. 2015, 63, 2963–2969. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, X.; Nelson, B.B.; Jin, E.; Sit, J.; Charney, N.; Yang, M.; Omary, M.B.; Yin, L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARalpha pathway. Hepatology 2018. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Xu, C.; Shao, S.; Liu, J.; Xing, W.; Xu, J.; Qin, C.; Li, C.; Hu, B.; Yi, S.; et al. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4alpha/CYP7A1 axis. J. Hepatol. 2015, 62, 1171–1179. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Xiao, Y.; Yong, Y.; Zhang, Z.; Li, S.; Zhou, T.; Yang, D.; Gao, P.; Xin, X. Hypolipidemic effects of Myrica rubra extracts and main compounds in C57BL/6j mice. Food Funct. 2016, 7, 3505–3515. [Google Scholar] [CrossRef]
Sample Availability: Samples of COS film coating tablets are available from the authors. |










| Index | NF | HF | AVT | CFTs-H | CFTs-M | CFTs-L |
|---|---|---|---|---|---|---|
| Serum (mmol/L) | ||||||
| TC (mmol/L) | 2.44 ± 0.52 ** | 4.92 ± 0.63 | 3.24 ± 0.37 ** | 3.91 ± 0.39 * | 4.14 ± 0.45 * | 4.24 ± 0.40 * |
| TG(mmol/L) | 0.95 ± 0.32 ** | 1.69 ± 0.15 | 1.06 ± 0.08 ** | 1.33 ± 0.25 ** | 1.47 ± 0.25 * | 1.53 ± 0.20 |
| HDL-C (mmol/L) | 1.51 ± 0.31 * | 0.82 ± 0.28 | 1.06 ± 0.32 | 1.06 ± 0.32 | 1.56 ± 0.29 | 0.96 ± 0.45 |
| LDL-C (mmol/L) | 1.11 ± 0.25 *** | 3.16 ± 0.25 | 1.89 ± 0.22 * | 2.43 ± 0.32 * | 2.61 ± 0.35 * | 2.80 ± 0.22 |
| AI | 0.96 ± 0.55 *** | 5.26 ± 1.77 | 1.79 ± 0.44 *** | 2.39 ± 0.18 *** | 2.78 ± 0.43 ** | 3.92 ± 0.75 ** |
| AST (U/L) | 83.06 ± 13.86 ** | 138.22 ± 27.98 | 134.02 ± 21.13 | 114.20 ± 16.78 * | 115.52 ± 15.65 | 118.46 ± 17.80 |
| ALT (U/L) | 44.88 ± 13.56 ** | 92.88 ± 11.35 | 85.52 ± 8.76 | 75.00 ± 17.62 * | 78.64 ± 19.76 | 85.18 ± 15.23 |
| Liver (mg/g) | ||||||
| TC | 2.66 ± 0.58 ## | 4.97 ± 0.64 | 3.44 ± 0.62 # | 3.83 ± 0.48 # | 4.24 ± 0.44 # | 4.28 ± 0.53 # |
| TG | 2.94 ± 0.28 # | 4.48 ± 0.54 | 3.84 ± 0.68 # | 4.02 ± 0.23 # | 4.07 ± 0.2 # | 3.98 ± 0.22 # |
| HDL-C | 1.35 ± 0.15 # | 0.9 ± 0.23 | 1.19 ± 0.13 | 1.13 ± 0.18 | 1.12 ± 0.1 | 1.03 ± 0.07 |
| LDL-C | 2.38 ± 0.35 ## | 3.73 ± 0.35 | 2.72 ± 0.37 ## | 3.44 ± 0.26 # | 3.55 ± 0.3 # | 3.64 ± 0.28 # |
| Fecal | ||||||
| TC | 6.53 ± 1.01 ** | 10.74 ± 1.48 | 11.67 ± 0.83 | 15.12 ± 1.69 ** | 14.60 ± 2.04 ** | 12.04 ± 1.94 * |
| TG | 6.40 ± 1.42 ** | 12.50 ± 0.96 | 13.63 ± 1.11 | 15.62 ± 1.80 * | 15.32 ± 2.04 * | 13.24 ± 2.72 |
| TBA | 1.83 ± 0.53 ** | 3.20 ± 0.91 | 2.90 ± 0.67 | 4.46 ± 1.25 * | 4.40 ± 1.25* | 4.58 ± 1.41 * |
| Primers | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CYP7A1 | ACCTGCCGGTACTAGACAGC | CAGGACATATTGT CGCGCCT |
| LDLR | GCCGACCTGACGAATTCCAG | ATCCGACCAGTCACGACAGT |
| LXRA | CTGCAACGGAGTTGTGGAAG | TCGCAGCTCAGCACATTGTA |
| SREBP2 | GGAGACCATGGAGACCCTCAC | AGACAATGGGACCTGGCTGAA |
| HMGCR | CCTCC ATTGAGATCCGGAGGA | ACAAAGAGGCCATGCATACGG |
| PPARα | TCTGA ACATTGGCGTTCGCA+ | TCCCTCAAGGGG ACA ACCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Hu, C.; Deng, X.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats. Molecules 2019, 24, 514. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030514
Yang D, Hu C, Deng X, Bai Y, Cao H, Guo J, Su Z. Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats. Molecules. 2019; 24(3):514. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030514
Chicago/Turabian StyleYang, Di, Canji Hu, Xiaoyi Deng, Yan Bai, Hua Cao, Jiao Guo, and Zhengquan Su. 2019. "Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats" Molecules 24, no. 3: 514. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030514