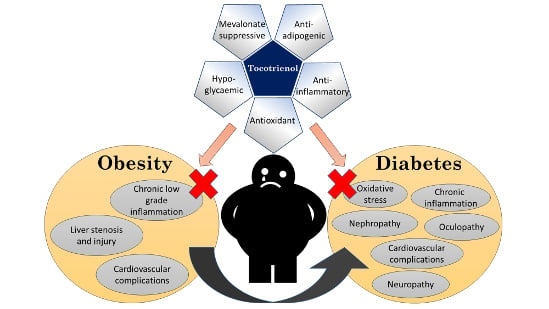
The Role of Tocotrienol in Protecting Against Metabolic Diseases
Abstract
:1. Introduction
2. Literature Search
3. Tocotrienol and Obesity
3.1. Anti-Obesity Properties of T3
3.2. Molecular Mechanism of Anti-Adipogenic Properties of T3
3.2.1. Suppression of Adipogenesis by T3
3.2.2. Pro-Apoptotic Effects of T3 on Adipocytes
3.3. Other Beneficial Effects of T3 on Obesity Management
3.3.1. Anti-Inflammatory Activity of T3
3.3.2. Effects of T3 on Glycemic Status and Insulin Sensitivity in Obesity Models
3.3.3. The Effects of T3 on Liver and Lipid Profile
3.3.4. Cardioprotective Effects of T3
4. Tocotrienol and Diabetes Mellitus
4.1. Antidiabetic Properties of T3 in Cell Lines and Diabetic Animals
4.2. Antidiabetic Activities of T3 in Human Studies
4.3. Effects of T3 in Diabetes-Mediated Lipid Abnormalities
4.4. Anti-Inflammatory Effects of T3 in Diabetic Models
4.5. Antioxidant Properties of T3 in Diabetic Animal Models
4.6. Effects of T3 in Diabetic Nephropathy
4.7. Effects of T3 in Diabetes-Related Cardiovascular Diseases
4.8. Effects of T3 in Diabetic Cataractogenesis
4.9. Effects of T3 in Diabetic Neuropathy
4.10. Effects of T3 on Skeletal Muscle of Diabetic Animals
5. Metabolites of T3 and Their Potential Metabolic Effects
6. Difference in the Metabolic Effect between T3 and Alpha-Tocopherol
7. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 May 2018).
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized? J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H. Oxidative stress in pancreatic beta cell regeneration. Oxid. Med. Cell Longev. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 1492. [Google Scholar] [CrossRef]
- McGuire, H.; Longson, D.; Adler, A.; Farmer, A.; Lewin, I. Management of type 2 diabetes in adults: Summary of updated nice guidance. BMJ 2016, 353, i1575. [Google Scholar] [CrossRef]
- Mitri, J.; Hamdy, O. Diabetes medications and body weight. Expert Opin. Drug Saf. 2009, 8, 573–584. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Vitamin E as an antiosteoporotic agent via receptor activator of nuclear factor kappa-b ligand signaling disruption: Current evidence and other potential research areas. Evid. Based Complement Alternat. Med. 2012, 2012, 747020. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, J.; Posada, L.R.; Kakuda, Y.; Xue, S.J. Separating tocotrienols from palm oil by molecular distillation. Food Rev. Int. 2008, 24, 376–391. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Bocci, F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 1998, 75, 1723–1727. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, X.; Marshall, M.; Chung, S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules 2016, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Radhakrishnan, A.K. Tocotrienol research: Past into present. Nutr. Rev. 2012, 70, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E as a potential interventional treatment for metabolic syndrome: Evidence from animal and human studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef]
- Burdeos, G.C.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol attenuates triglyceride accumulation in hepg2 cells and f344 rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef]
- Ali, S.F.; Nguyen, J.C.; Jenkins, T.A.; Woodman, O.L. Tocotrienol-rich Tocomin attenuates oxidative stress and improves endothelium-dependent relaxation in aortae from rats fed a high-fat western diet. Front. Cardiovasc. Med. 2016, 3, 39. [Google Scholar] [CrossRef]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar] [PubMed]
- Torabi, S.; Yeganehjoo, H.; Shen, C.L.; Mo, H. Peroxisome proliferator-activated receptor γ down-regulation mediates the inhibitory effect of d-δ-tocotrienol on the differentiation of murine 3T3-F442a preadipocytes. Nutr. Res. 2016, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.G.; Song, R.P.; Wang, Y.; Ge, S.; Zhang, Y.H.; Wang, H.X.; Liu, J.; Liu, L.X. R-tocotrienol inhibits cell proliferation of human gastric cancer by regulating nuclear factor-κB activity. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3t3-l1 cells: Size and optical density distribution. Eur. J. Histochem. 2013, 57, e24. [Google Scholar] [CrossRef] [PubMed]
- Burdeos, G.C.; Nakagawa, K.; Abe, T.; Kimura, F.; Miyazawa, T. Tocotrienol modulates crucial lipid metabolism-related genes in differentiated 3T3-L1 preadipocytes. Food Funct. 2014, 5, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ha, J.H.; Okla, M.; Chung, S. Activation of autophagy and AMPK by gamma-tocotrienol suppresses the adipogenesis in human adipose derived stem cells. Mol. Nutr. Food Res. 2014, 58, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Huang, G.Y.; Ng, L.T. γ-tocotrienol induced cell cycle arrest and apoptosis via activating the bax-mediated mitochondrial and ampk signaling pathways in 3T3-L1 adipocytes. Food Chem. Toxicol. 2013, 59, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Burdeos, G.C.; Nakagawa, K.; Watanabe, A.; Kimura, F.; Miyazawa, T. γ-tocotrienol attenuates triglyceride through effect on lipogenic gene expressions in mouse hepatocellular carcinoma Hepa 1-6. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Zaiden, N.; Yap, W.N.; Ong, S.; Xu, C.H.; Teo, V.H.; Chang, X.W.; Nesaretnam, K.; Shiba, S.; Yap, Y.L. Gamma delta tocotrienols reduce hepatic triglyceride synthesis and VLDL secretion. J. Atheroscler. Thromb. 2010, 17, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yagiz, Y.; Xu, C.; Lu, J.; Chung, S.; Marshall, M.R. Muscadine grape seed oil as a novel source of tocotrienols to reduce adipogenesis and adipocyte inflammation. Food Funct. 2015, 6, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Candiracci, M.; Justo, M.L.; Castano, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and m1 macrophage recruitment. Int. J. Obes. 2014, 39, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Poudyal, H.; Ward, L.C.; Brown, L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients 2012, 4, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Ima-Nirwana, S.; Suhaniza, S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Azwan, K.; Farihah, H.S.; Fairus, A.; Elvy, M.R. Effect of palm oil (Elaeis guineensis) tocotrienols on mesenteric adipose tissue deposition and the expression of 11β-hydroxysteroid dehydrogenase type 1 enzyme (11β-HSD1) in adrenalectomized rats treated with dexamethasone. Clin. Ter. 2015, 166, 99–104. [Google Scholar]
- Betik, A.C.; Aguila, J.; McConell, G.K.; McAinch, A.J.; Mathai, M.L. Tocotrienols and whey protein isolates substantially increase exercise endurance capacity in diet -induced obese male Sprague-Dawley rats. PLoS ONE 2016, 11, e0152562. [Google Scholar] [CrossRef]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef]
- Matsunaga, T.; Shoji, A.; Gu, N.; Joo, E.; Li, S.; Adachi, T.; Yamazaki, H.; Yasuda, K.; Kondoh, T.; Tsuda, K. γ-tocotrienol attenuates TNF-α-induced changes in secretion and gene expression of MCP-1, IL-6 and adiponectin in 3T3-L1 adipocytes. Mol. Med. Rep. 2012, 5, 905–909. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-tocotrienol inhibits nuclear factor-κB signaling pathway through inhibition of receptor-interacting protein and Tak1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Q. γ-tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPβ and NF-κB in macrophages. J. Nutr. Biochem. 2013, 24, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wang, W.; Okla, M.; Kang, I.; Moreau, R.; Chung, S. Suppression of NLRP3 inflammasome by γ-tocotrienol ameliorates type 2 diabetes. J. Lipid Res. 2016, 57, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, Y. Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J. Nutr. Biochem. 2018, 57, 77–85. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Duval, C.; Muller, M.; Kersten, S. PPARs, obesity, and inflammation. PPAR Res. 2007, 2007, 95974. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chawla, A.; Urbiztondo, N.; Boisvert, W.A.; Evans, R.M.; Curtiss, L.K. Transcriptional repression of atherogenic inflammation: Modulation by PPARδ. Science 2003, 302, 453–457. [Google Scholar] [CrossRef]
- Magosso, E.; Ansari, M.A.; Gopalan, Y.; Shuaib, I.L.; Wong, J.W.; Khan, N.A.; Abu Bakar, M.R.; Ng, B.H.; Yuen, K.H. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: A randomised placebo-controlled clinical trial. Nutr. J. 2013, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFκB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Rashid Khan, M.; Siddiqui, W.A. Comparative hypoglycemic and nephroprotective effects of tocotrienol rich fraction (TRF) from palm oil and rice bran oil against hyperglycemia induced nephropathy in type 1 diabetic rats. Chem. Biol. Interact. 2010, 188, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Ahsan, H.; Khan, M.R.; Siddiqui, W.A. Protective effects of tocotrienols against lipid-induced nephropathy in experimental type-2 diabetic rats by modulation in TGF-β expression. Toxicol. Appl. Pharmacol. 2013, 273, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Wan Nazaimoon, W.M.; Khalid, B.A.K. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays. J. Pathol. 2002, 24, 77–82. [Google Scholar] [PubMed]
- Budin, S.B.; Othman, F.; Louis, S.R.; Bakar, M.A.; Das, S.; Mohamed, J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2009, 64, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Bishnoi, M.; Tiwari, V.; Chopra, K. Suppression of NF-κB signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009, 92, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Abdul-Rahman, M.; Al-Wahaibi, N.; Mohammed, J. Tocotrienol-rich fraction from palm oil prevents oxidative damage in diabetic rats. Sultan Qaboos Univ. Med. J. 2014, 14, e95–e103. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Cheng, H.H. A rice bran oil diet increases LDL-receptor and HMG-Coa reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Nutr. 2006, 136, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.L.; Jantan, I.; Chua, K.H.; Lam, K.W.; Rullah, K.; Aluwi, M.F. Effects of tocotrienols on insulin secretion-associated genes expression of rat pancreatic islets in a dynamic culture. Front. Pharmacol. 2016, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.T.; Geny, C.; Blanchard, P.; Rouger, C.; Tonnerre, P.; Charreau, B.; Rakolomalala, G.; Randriamboavonjy, J.I.; Loirand, G.; Pacaud, P.; et al. Advanced glycation inhibition and protection against endothelial dysfunction induced by coumarins and procyanidins from mammea neurophylla. Fitoterapia 2014, 96, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Fronseca, V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am. J. Med. 2003, 115, 42S–48S. [Google Scholar] [CrossRef]
- Ko, K.D.; Kim, K.K.; Lee, K.R. Does weight gain associated with thiazolidinedione use negatively affect cardiometabolic health? J. Obes. Metab. Syndr. 2017, 26, 102–106. [Google Scholar] [CrossRef]
- Kaup, R.M.; Khayyal, M.T.; Verspohl, E.J. Antidiabetic effects of a standardized Egyptian rice bran extract. Phytother. Res. 2013, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Onodera, A.; Saito, E.; Tanabe, M.; Yajima, K.; Takahashi, J.; Nguyen, V.C. Effect of astaxanthin in combination with α-tocopherol or ascorbic acid against oxidative damage in diabetic ods rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 329–334. [Google Scholar] [CrossRef]
- Chou, T.W.; Ma, C.Y.; Cheng, H.H.; Chen, Y.Y.; Lai, M.H. A rice bran oil diet improves lipid abnormalities and suppress hyperinsulinemic responses in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Clin. Biochem. Nutr. 2009, 45, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.K.; Kontto, J.P.; Mannisto, S.; Albanes, D.; Virtamo, J. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur. J. Clin. Nutr. 2011, 65, 590–597. [Google Scholar] [CrossRef]
- Vafa, M.; Haghighat, N.; Moslehi, N.; Eghtesadi, S.; Heydari, I. Effect of tocotrienols enriched canola oil on glycemic control and oxidative status in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2015, 20, 540–547. [Google Scholar] [CrossRef]
- Hor, C.P.; Fung, W.Y.; Ang, H.A.; Lim, S.C.; Kam, L.Y.; Sim, S.W.; Lim, L.H.; Choon, W.Y.; Wong, J.W.; Ch’ng, A.S.H.; et al. Efficacy of oral mixed tocotrienols in diabetic peripheral neuropathy: A randomized clinical trial. JAMA Neurol. 2018, 75, 444–452. [Google Scholar]
- Wan Nazaimoon, W.M.; Sakinah, O.; Gapor, A.; Khalid, B.A.K. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr. Res. 1996, 16, 1901–1911. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Brinkworth, G.D.; Thompson, C.H.; Abeywardena, M.Y. Short term effects of palm-tocotrienol and palm-carotenes on vascular function and cardiovascular disease risk: A randomised controlled trial. Atherosclerosis 2016, 254, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ozder, A. Lipid profile abnormalities seen in t2dm patients in primary healthcare in Turkey: A cross-sectional study. Lipid Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G. Pathophysiology of diabetic dyslipidemia: Implications for atherogenesis and treatment. Clin. Lipidol. 2011, 6, 401–411. [Google Scholar] [CrossRef]
- Pathak, R.; Ghosh, S.P.; Zhou, D.; Hauer-Jensen, M. The vitamin E analog gamma-tocotrienol (GT3) and statins synergistically up-regulate endothelial thrombomodulin (TM). Int. J. Mol. Sci. 2016, 17, E1937. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Abdul-Majeed, S.; Mohamed, N.; Ima-Nirwana, S. The effects of tocotrienol and lovastatin co-supplementation on bone dynamic histomorphometry and bone morphogenetic protein-2 expression in rats with estrogen deficiency. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Soelaiman, I.N.; Pang, K.L. Tocotrienol and its role in chronic diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B., Eds.; Springer: Basel, Switzerland, 2016; Volume 928, pp. 97–130. [Google Scholar]
- Haghighat, N.; Vafa, M.; Eghtesadi, S.; Heidari, I.; Hosseini, A.; Rostami, A. The effects of tocotrienols added to canola oil on microalbuminuria, inflammation, and nitrosative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Int. J. Prev. Med. 2014, 5, 617–623. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Yang, H.; Jin, X.; Kei Lam, C.W.; Yan, S.K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V.; Mohr, S.; Grant, M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 2008, 57, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br. J. Pharmacol. 2009, 156, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Matnor, N.A.; Iyer, A.; Brown, L. A regenerative antioxidant protocol of vitamin E and α-lipoic acid ameliorates cardiovascular and metabolic changes in fructose-fed rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 120801. [Google Scholar] [CrossRef] [PubMed]
- Muharis, S.P.; Top, A.G.; Murugan, D.; Mustafa, M.R. Palm oil tocotrienol fractions restore endothelium dependent relaxation in aortic rings of streptozotocin-induced diabetic and spontaneously hypertensive rats. Nutr. Res. 2010, 30, 209–216. [Google Scholar] [CrossRef]
- Kamat, J.P.; Sarma, H.D.; Devasagayam, T.P.; Nesaretnam, K.; Basiron, Y. Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol. Cell Biochem. 1997, 170, 131–137. [Google Scholar] [CrossRef]
- Dokken, B.B. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008, 21, 160–165. [Google Scholar] [CrossRef]
- Meigs, J.B. Epidemiology of type 2 diabetes and cardiovascular disease: Translation from population to prevention: The Kelly West Award lecture 2009. Diabetes Care 2010, 33, 1865–1871. [Google Scholar] [CrossRef]
- Abdul Nasir, N.A.; Agarwal, R.; Vasudevan, S.; Tripathy, M.; Alyautdin, R.; Mohd Ismail, N. Effects of topically applied tocotrienol on cataractogenesis and lens redox status in galactosemic rats. Mol. Vis. 2014, 20, 822–835. [Google Scholar] [PubMed]
- Abdul Nasir, N.A.; Agarwal, R.; Sheikh Abdul Kadir, S.H.; Vasudevan, S.; Tripathy, M.; Iezhitsa, I.; Mohammad Daher, A.; Ibrahim, M.I.; Mohd Ismail, N. Reduction of oxidative-nitrosative stress underlies anticataract effect of topically applied tocotrienol in streptozotocin-induced diabetic rats. PLoS ONE 2017, 12, e0174542. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.K.; Ridlington, J.; Leonard, S.; Vaule, H.; Traber, M.G. Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids 2001, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Schmölz, L.; Birringer, M.; Lorkowski, S.; Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016, 7, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Pfluger, P.; Kluth, D.; Landes, N.; Brigelius-Flohe, R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002, 132, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Freiser, H.; Jiang, Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 2009, 139, 884–889. [Google Scholar] [CrossRef]
- Grammas, P.; Hamdheydari, L.; Benaksas, E.J.; Mou, S.; Pye, Q.N.; Wechter, W.J.; Floyd, R.A.; Stewart, C.; Hensley, K. Anti-inflammatory effects of tocopherol metabolites. Biochem. Biophys. Res. Commun. 2004, 319, 1047–1052. [Google Scholar] [CrossRef]
- Varga, Z.; Kosaras, E.; Komodi, E.; Katko, M.; Karpati, I.; Balla, J.; Paragh, G.; Aisa, M.C.; Galli, F. Effects of tocopherols and 2,2′-carboxyethyl hydroxychromans on phorbol-ester-stimulated neutrophils. J. Nutr. Biochem. 2008, 19, 320–327. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Kiyose, C.; Ohmori, R.; Saito, H.; Taguchi, C.; Kishimoto, Y.; Machida, N.; Hasegawa, M.; Yoshioka, E.; Saita, E.; et al. The coantioxidative effects of carboxyethyl-6-hydroxychromans and alpha-tocopherol. J. Nutr. Sci. Vitaminol. (Tokyo) 2007, 53, 301–305. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Jiang, Q. Natural forms of vitamin e and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 2011, 186, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wallert, M.; Mosig, S.; Rennert, K.; Funke, H.; Ristow, M.; Pellegrino, R.M.; Cruciani, G.; Galli, F.; Lorkowski, S.; Birringer, M. Long-chain metabolites of alpha-tocopherol occur in human serum and inhibit macrophage foam cell formation in vitro. Free Radic Biol. Med. 2014, 68, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Nor, R.M.; Fairus, S.; Sundram, K. Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of α-tocopherol. Am. J. Clin. Nutr. 2006, 84, 835–842. [Google Scholar]
- Fu, J.-Y.; Che, H.-L.; Tan, D.M.-Y.; Teng, K.-T. Bioavailability of tocotrienols: Evidence in human studies. Nutr. Metab. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin e analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Tanaka-Yachi, R.; Takahashi-Muto, C.; Adachi, K.; Tanimura, Y.; Aoki, Y.; Koike, T.; Kiyose, C. Promoting effect of alpha-tocopherol on beige adipocyte differentiation in 3T3-L1 cells and rat white adipose tissue. J. Oleo Sci. 2017, 66, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Cunha, C.A.; Ribeiro, E.B.; do Nascimento, C.O.; Oyama, L.M.; Mota, J.F. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Lirangi, M.; Meydani, M.; Zingg, J.M.; Azzi, A. Alpha-tocopheryl-phosphate regulation of gene expression in preadipocytes and adipocytes. Biofactors 2012, 38, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, W.; Doi, W.; Takemoto, K.; Ishihara, K.; Wang, D.H.; Sugiyama, H.; Oda, S.; Masuoka, N. Effect of vitamin E on alloxan-induced mouse diabetes. Clin. Biochem. 2013, 46, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Doi, W.; Masuoka, N. Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Biochim. Biophys. Acta 2016, 1862, 647–650. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.; Sundell, J.R.; Mannisto, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia 2008, 51, 47–53. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.K.; Kontto, J.P.; Mannisto, S.; Albanes, D.; Virtamo, J.R. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: Results of the ATBC study. Ann. Med. 2010, 42, 178–186. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.M.; Rondo, P.H.; Luzia, L.A.; D’Abronzo, F.H.; Illison, V.K. The effects of lipoic acid and alpha-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Wu, J.H.; Clarke, M.W.; Puddey, I.B.; Burke, V.; Croft, K.D.; Hodgson, J.M. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. J. Hypertens 2007, 25, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Ward, N.C.; Indrawan, A.P.; Almeida, C.A.; Hodgson, J.M.; Proudfoot, J.M.; Puddey, I.B.; Croft, K.D. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin. Chem. 2007, 53, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef] [PubMed]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm tocotrienol exerted better antioxidant activities in bone than α-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nazrun, A.; Khairunnur, A.; Norliza, M.; Norazlina, M.; Ima Nirwana, S. Effects of palm tocotrienol on oxidative stress and bone strength in ovariectomised rats. Med. Health 2008, 3, 83–90. [Google Scholar]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K. Vitamin e: Regulatory redox interactions. IUBMB Life 2019, in press. [Google Scholar] [CrossRef]
- Norazlina, M.; Hermizi, H.; Faizah, O.; Ima-Nirwana, S. Vitamin e reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch. Med. Sci. 2010, 6, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.S.; Khalid, B.A.; Luke, D.A.; Ima Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef]
- Shibata, A.; Kawakami, Y.; Kimura, T.; Miyazawa, T.; Nakagawa, K. Alpha-tocopherol attenuates the triglyceride- and cholesterol-lowering effects of rice bran tocotrienol in rats fed a western diet. J. Agric. Food Chem. 2016, 64, 5361–5366. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A.; Peterson, D.M.; Elson, C.E. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in chickens. J. Nutr. 1996, 126, 389–394. [Google Scholar] [CrossRef]
- Khor, H.T.; Ng, T.T. Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG-CoA reductase activity. Int. J. Food Sci. Nutr. 2000, 51, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Ima-Nirwana, S.; Nurshazwani, Y.; Nazrun, A.S.; Norliza, M.; Norazlina, M. Subacute and subchronic toxicity studies of palm vitamin E in mice. J. Pharmacol. Toxicol. 2011, 6, 166–173. [Google Scholar] [CrossRef]
- Nakamura, H.; Furukawa, F.; Nishikawa, A.; Miyauchi, M.; Son, H.Y.; Imazawa, T.; Hirose, M. Oral toxicity of a tocotrienol preparation in rats. Food Chem. Toxicol. 2001, 39, 799–805. [Google Scholar] [CrossRef]



| T3 Isoform | Treatment Condition and Population | Main Outcomes | References |
|---|---|---|---|
| Dietary intake of T3 | The risk of diabetes and dietary intake of T3 was investigated via The Finnish Mobile Health Examination survey (cohort study) on 4504 healthy subjects |
| [68] |
| The risk of diabetes and dietary intake of T3 was investigated via ATBC cohort study on 25,505 healthy subjects |
| [69] | |
| T3 (unknown T3 composition) | Oral mixed T3 (400 mg/day) capsules were supplied to 229 diabetic patients with diabetic peripheral neuropathy syndromes (VENUS study) |
| [71] |
| Palmvitee (16% T3) | Palmvitee (1800 mg) or refined palm oil capsules were provided to 32 T2DM patients for 60 days, followed by 60 days washout period and then crossed over the supplementation for another 60 days |
| [72] |
| TRF (14.6% α-T3, 2.2% β-T3, 38.8% γ-T3, and 2.4% unidentified T3) | TRF treatment (6 mg/kg bw/day) was supplied to 19 T2DM patients for 60 days (RCT study) |
| [73] |
| TRF (24.5% α-T3, 3.5% β-T3, 35.4% γ-T3, 12.7% δ-T3, and 23.9% α-tocopherol) | 552 mg/day of TRF capsules was supplied to 86 T2DM patients with impaired vascular function for 8 weeks (RCT study) |
| [74] |
| T3 mixture (13.2% α-T3, 16.6% γ-T3, 8.6% others T3, and 16% α-tocopherol) | 200 mg/day of T3 mixture was supplied to 44 T2DM patients for 8 weeks (RCT study) |
| [80] |
| 200 mg/day of T3 mixture was supplied to 45 T2DM patients for 8 weeks (RCT study) |
| [70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-L.; Chin, K.-Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050923
Pang K-L, Chin K-Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules. 2019; 24(5):923. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050923
Chicago/Turabian StylePang, Kok-Lun, and Kok-Yong Chin. 2019. "The Role of Tocotrienol in Protecting Against Metabolic Diseases" Molecules 24, no. 5: 923. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050923