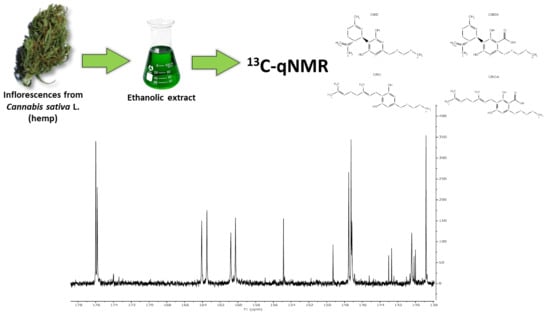
Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp)
Abstract
:1. Introduction
2. Results and Discussion
2.1. NMR Spectroscopic Data of Extracts from Hemp Inflorescences
- low sensitivity, due to the lower 13C isotopic abundance (1%) in comparison to 1H;
- carbons typically have long T1 relaxation time; in this way, signals could not reach their full intensity;
- the 13C signal intensity is affected by the Nuclear Overhauser Enhancement (NOE) effect, that depends on 13C type (from primary to quaternary).
2.2. 13C-qNMR Method Validation
2.3. Quantitative Analysis of Hemp Inflorescences
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Hemp Plant Material
3.3. Sample Preparation
3.4. NMR Spectroscopy and Spectra Acquisition Procedures
3.5. 13C-qNMR Procedure
3.6. HPLC Analysis
3.7. Method Validation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Appendino, G.; Taglialatela-Scafati, O. Cannabinoids: Chemistry and Medicine. In Natural Products; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 3415–3435. [Google Scholar]
- Karlov, G.I.; Razumova, O.V.; Alexandrov, O.S.; Divashuk, M.G.; Kroupin, P.Y. Classical and Molecular Cytogenetics of Cannabis sativa L. In Cannabis sativa L.-Botany and Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; pp. 385–394. [Google Scholar]
- ElSohly, M.A.; Lata, H.; Chandra, S. Cannabis sativa L.-botany and biotechnology; Springer: Berlin, Germany, 2017; ISBN 9783319545646. [Google Scholar]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, L. Scientifically sound guidelines for THC in food in Europe. Available online: http://eiha.org/media/2015/08/15-07-24-Report-Scientifically-Safe-Guidelines-THC-Food-nova-EIHA.pdf (accessed on 5 February 2019).
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S.; Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; et al. New methods for the comprehensive nalysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [PubMed]
- Happyana, N.; Kayser, O. Monitoring metabolite profiles of Cannabis sativa L. trichomes during flowering period using 1H NMR-based metabolomics and real-time PCR. Planta Med. 2016, 82, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; ElSohly, M.A.; Sultana, G.N.N.; Mehmedic, Z.; Hossain, C.F.; Chandra, S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem. Anal. 2005, 16, 45–48. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. PKS Activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol. 2008, 49, 1767–1782. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Omar, J.; Navarro, P.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7549–7560. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Moreira, F.A.; Guimarães, F.S. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Brazilian J. Med. Biol. Res. 2006, 39, 421–429. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: their chemistry and role against oxidative stress, inflammation, and cancer. Biomed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Corsi, L.; Pellati, F.; Brighenti, V.; Plessi, N.; Benvenuti, S. Chemical composition and in vitro neuroprotective activity of fibre-type Cannabis sativa L. (hemp). Curr. Bioact. Compd. 2019, 15. in press. [Google Scholar] [CrossRef]
- Eubanks, L.M.; Rogers, C.J.; Beuscher, A.E.; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A Molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 2006, 3, 773–777. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure−activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Cascio, M.; Gauson, L.; Stevenson, L.; Ross, R.; Pertwee, R. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Takeda, S.; Okajima, S.; Miyoshi, H.; Yoshida, K.; Okamoto, Y.; Okada, T.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; Aramaki, H. Cannabidiolic acid, a major cannabinoid in fiber-type Cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration. Toxicol. Lett. 2012, 214, 314–319. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic compounds and antioxidant activity of cold-pressed seed oil from finola cultivar of Cannabis sativa L. Phyther. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Radwan, M.; Ross, S.; Slade, D.; Ahmed, S.; Zulfiqar, F.; ElSohly, M. Isolation and characterization of new Cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267–272. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: from crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Chauthe, S.K.; Sharma, R.J.; Aqil, F.; Gupta, R.C.; Singh, I.P. Quantitative NMR: an applicable method for quantitative analysis of medicinal plant extracts and herbal products. Phytochem. Anal. 2012, 23, 689–696. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Wong, Y.-I. Nuclear magnetic resonance spectroscopic determination of α- and β-acid homolog composition in hops. J. Agric. Food Chem. 1975, 23, 1201–1204. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.-N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napolitano, J.G. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef]
- Bertelli, D.; Brighenti, V.; Marchetti, L.; Reik, A.; Pellati, F. Nuclear magnetic resonance and high-performance liquid chromatography techniques for the characterization of bioactive compounds from Humulus lupulus L. (hop). Anal. Bioanal. Chem. 2018, 410, 3521–3531. [Google Scholar] [CrossRef]
- Protti, M.; Brighenti, V.; Battaglia, M.R.; Anceschi, L.; Pellati, F.; Mercolini, L. Cannabinoids from Cannabis sativa L.: A new tool based on HPLC− DAD−MS/MS for a rational use in medicinal chemistry. ACS Med. Chem. Lett. 2019, in press. [Google Scholar] [CrossRef]
- Plant Variety Catalogues, Databases & Information Systems. Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases_en (accessed on 12 February 2019).
Sample Availability: Hemp samples are available from the authors. |





| CBD | CBDA | CBG | CBGA | |
|---|---|---|---|---|
| 1H a | 1H a | 1H a | 1H a | |
| 1 | 3.86 (1H, m, 11.8 Hz) | 3.88 (1H, m, 11.0 Hz) | 1.33 (2H, d, 7.0 Hz) | 1.79 (2H, d, 7.4 Hz) |
| 2 | 5.55 (1H, s) | 5.55 (1H, s) | 5.27 (1H, t, 7.0 Hz) | 5.27 (1H, t, 7.0 Hz) |
| 3 | - | - | - | - |
| 4 | 2.10 (1H, m); 2.20 (1H, m) | 2.10 (1H, m) 2.20 (1H, m) | 2.04 (2H, t, 6.6 Hz) | 2.04 (2H, t, 6.6 Hz) |
| 5 | 1.84 (2H, q, 3.0 Hz) | 1.86 (2H, q, 3.0 Hz) | 2.07 (2H, q, 6.5 Hz) | 2.07 (2H, q, 6.5 Hz) |
| 6 | 2.40 (1H, m) | 2.40 (1H, m) | 5.56 (1H, m) | 5.05 (1H, t, 6.6 Hz) |
| 7 | 1.79 (3H, s) | 1.79 (3H, s) | 1.79 (3H, s) | 1.80 (3H, s) |
| 8 | - | - | - | - |
| 9 | 1.66 (3H, s) | 1.72 (3H, s) | 1.68 (1H, s) | 1.67 (3H, s) |
| 10 | 4.40 (2H, m) | 4.54 (2H, m) | 1.58 (1H, s) | 1.58 (3H, s) |
| 1’ | - | - | - | - |
| 2’ | - | - | - | - |
| 3’ | 6.26 (1H, brs) | - | 6.0 (1H, s) | - |
| 4’ | - | - | - | - |
| 5’ | 6.16 (1H, brs) | 6.26 (1H, s) | 6.24 (1H, s) | 6.23 (1H, s) |
| 6’ | - | - | - | - |
| 1’’ | 2.42 (2H, t, 7.5 Hz) | 2.42 (2H, t, 7.5 Hz) | 2.44 (2H, t, 7.5 Hz) | 2.88 (2H, t, 7.6 Hz) |
| 2’’ | 1.57 (2H, m) | 1.58 (2H, m) | 1.54 (2H, m) | 2.10 (2H, m) |
| 3’’ | 1.30 (2H, m) | 1.33 (2H, m) | 1.56 (2H, m) | 1.32 (2H, m) |
| 4’’ | 1.31 (2H, m) | 1.34 (2H, m) | 1.57 (2H, m) | 1.32 (2H, m) |
| 5’’ | 0.89 (3H, t, 6.8 Hz) | 0.90 (3H, t, 6.8 Hz) | 0.88 (3H, t, 6.9 Hz) | 0.89 (3H, t, 6.9 Hz) |
| CBD | CBDA | CBG | CBGA | |||||
|---|---|---|---|---|---|---|---|---|
| 13C | T1 s | 13C | T1 s | 13C | T1 s | 13C | T1 s | |
| 1 | 37.0 * | 1.32 | 36.7 * | 1.51 | 22.5 * | 1.35 | 25.7 * | 1.54 |
| 2 | 124.3 * | 1.20 | 124.0 * | 1.35 | 121.8 * | 1.89 | 121.5 * | 1.92 |
| 3 | 139.9 | 5.52 | 140.3 | - | 140.2 | 6.02 | 138.8 | - |
| 4 | 31.5 | 2.10 | 31.3 | - | 39.7 | 3.00 | 39.7 | - |
| 5 | 28.4 | 0.97 | 27.8 | - | 26.3 | 1.02 | 26.4 | - |
| 6 | 46.2 * | 1.90 | 46.6 * | 1.91 | 123.8 | 2.05 | 123.8 | - |
| 7 | 23.4 * | 1.41 | 23.7 * | 1.61 | 16.0 * | 1.23 | 16.2 * | 1.40 |
| 8 | 149.9 | 5.52 | 147.2 | - | 132.0 | 5.50 | 131.7 | - |
| 9 | 20.3 | 2.67 | 18.9 | - | 23.4 | 2.68 | 23.4 | - |
| 10 | 110.8 * | 0.96 | 111.3 * | 1.10 | 17.6 | 1.03 | 17.8 | - |
| 1’ | 113.8 | 8.08 | 114.4 | - | 110.7 | 6.66 | 111.1 | - |
| 2’ | 156.0 | - | 164.1 ** | 2.5 | 154.9 | - | 163.5 ** | 2.65 |
| 3’ | 108.3 | - | 103.1 | - | 108.3 | - | 103.6 | - |
| 4’ | 142.9 | - | 147.2 | - | 142.7 | - | 147.5 | - |
| 5’ | 108.3 | - | 111.7 | - | 108.3 | - | 111.8 | - |
| 6’ | 153.9 | - | 160.1 ** | 2.7 | 154.9 | - | 160.8 ** | 2.81 |
| 1’’ | 35.5 * | 1.95 | 35.4 * | 1.95 | 35.6 * | 1.23 | 36.3 * | 1.25 |
| 2’’ | 30.4 * | 1.12 | 29.7 * | 1.12 | 30.8 * | 1.06 | 30.2 * | 1.10 |
| 3’’ | 30.7 * | 1.47 | 30.2 * | 1.47 | 31.5 * | 1.50 | 31.4 * | 1.52 |
| 4’’ | 22.5 | 3.82 | 22.5 | 3.88 | 22.5 | 3.90 | 22.5 | 3.92 |
| 5’’ | 14.1 | 3.19 | 14.1 | 3.23 | 14.1 | 3.20 | 14.1 | 3.36 |
| -COOH | - | - | 175.3 ** | 3.1 | - | - | 176.0 ** | 3.11 |
| CBD | CBDA | CBG | CBGA | |||||
|---|---|---|---|---|---|---|---|---|
| qNMR | HPLC * | qNMR | HPLC * | qNMR | HPLC | qNMR | HPLC | |
| Antal | 2.2 ± 0.2 | 2.4 ± 0.1 | 15.7 ± 0.5 | 15.5 ± 0.7 | <LOQ b | 0.3 a | 1.2 ± 0.2 | 0.6 a |
| Carma | 5.8 ± 0.4 | 6.0 ± 0.3 | <LOQb | 2.2 ± 0.1 | <LOQ b | 0.5 ± 0.1 | <LOQ b | 0.4 a |
| Carmagnola | 3.0 ± 0.4 | 3.3 ± 0.3 | 17.3 ± 0.6 | 16.7 ± 1.2 | <LOQ b | 0.1 a | 1.2 ± 0.1 | 0.7 ± 0.1 |
| China | 8.0 ± 0.5 | 8.4 ± 0.1 | 18.3 ± 0.7 | 17.2 ± 0.8 | <LOQ b | 0.2 ± 0.1 | <LOQ b | 0.5 ± 0.1 |
| Codimono | 9.3 ± 0.6 | 9.8 ± 0.3 | 1.1 ± 0.2 | 2.9 ± 0.3 | <LOQ b | 0.1 a | <LOQ b | < LOQ d |
| Fibrante | 6.6 ± 0.5 | 7.9 ± 0.5 | 14.7 ± 0.3 | 14.5 ± 1.1 | <LOQ b | 0.2 a | <LOQ b | 0.4 a |
| Futura | 2.8 ± 0.2 | 3.3 ± 0.1 | 34.0 ± 0.6 | 33.8 ± 0.3 | <LOQ b | <LOQ c | 1.7 ± 0.3 | 1.3 ± 0.1 |
| Santhica | 2.1 ± 0.1 | 2.3 ± 0.2 | 18.7 ± 0.8 | 17.3 ± 2.4 | 1.2 ± 0.1 | 1.4 a | 9.4 ± 0.4 | 9.9 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, L.; Brighenti, V.; Rossi, M.C.; Sperlea, J.; Pellati, F.; Bertelli, D. Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 1138. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24061138
Marchetti L, Brighenti V, Rossi MC, Sperlea J, Pellati F, Bertelli D. Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). Molecules. 2019; 24(6):1138. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24061138
Chicago/Turabian StyleMarchetti, Lucia, Virginia Brighenti, Maria Cecilia Rossi, Johanna Sperlea, Federica Pellati, and Davide Bertelli. 2019. "Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp)" Molecules 24, no. 6: 1138. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24061138