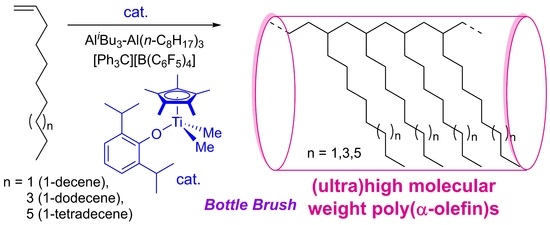
Synthesis of Ultrahigh Molecular Weight Polymers with Low PDIs by Polymerizations of 1-Decene, 1-Dodecene, and 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polymerization of 1-Decene and 1-Dodecene by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–Borate Catalyst
2.2. Polymerization of 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–Borate Catalyst
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. A tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. Engl. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.D.; Mountford, P. Transition metal imido compounds as Ziegler—Natta olefin polymerisation catalysts. Adv. Synth. Catal. 2005, 347, 355–366. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI Catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef]
- Gladysz, J.A. Frontiers in Metal-Catalyzed Polymerization: Designer Metallocenes, Designs on New Monomers, Demystifying MAO, Metathesis Déshabillé. Chem. Rev. 2000, 100, 1167–1168. [Google Scholar] [CrossRef]
- Milani, B.; Claver, C. (Eds.) Metal-Catalysed Polymerisation (special issue). Dalton Trans. 2009, 41, 8769–9076. [Google Scholar]
- Liu, B.; Terano, M.; Busico, V.; Wong, W.-Y.; Tang, Y. (Eds.) Metal-Catalyzed Polymerization of Olefins (special issue). J. Organomet. Chem. 2015, 798, 291–436. [Google Scholar]
- Osakada, K. (Ed.) Organometallic Reactions and Polymerization; Lecture Notes in Chemistry 85; Springer: Berlin, Germany, 2014. [Google Scholar]
- Hoff, R. (Ed.) Handbook of Transition Metal Polymerization Catalysts, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Choi, H.J.; Jhon, M.S. Polymer-induced turbulent drag reduction. Ind. Eng. Chem. Res. 1996, 35, 2993–2998. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E.; Tavtorkin, A.V. Polyolefin drag reducing agents. Petrol. Chem. 2016, 56, 775–787. [Google Scholar] [CrossRef]
- Brostow, W. Drag reduction in flow: Review of applications, mechanism and prediction. J. Ind. Eng. Chem. 2008, 14, 409–416. [Google Scholar] [CrossRef]
- Cuenca, F.G.; Marín, M.G.; Folgueras Díaz, M.B. Energy-savings modeling of oil pipelines that use drag-reducing additives. Energy Fuels 2008, 22, 3293–3298. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Younker, J.M.; Norman, A.I.; Schaefer, J.J.; Hagadorn, J.R.; Throckmorton, J.A. Microstructure of crystallizable α-olefin molecular bottlebrushes: Isotactic and atactic poly(1-octadecene). Macromolecules 2018, 51, 872–883. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Hagadorn, J.R.; Throckmorton, J.A. Highly entangled α-olefin molecular bottlebrushes: Melt structure, linear rheology, and interchain friction mechanism. Macromolecules 2018, 51, 6958–6966. [Google Scholar] [CrossRef]
- Paturej, J.; Sheiko, S.S.; Panyukov, S.; Rubinstein, M. Molecular structure of bottlebrush polymers in melts. Sci. Adv. 2016, 2, e1601478. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Cao, Z.; Wang, Z.; Sheiko, S.S.; Dobrynin, A.V. Combs and Bottlebrushes in a Melt. Macromolecules 2017, 50, 3430–3437. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of higher α-olefins with metallocene/MAO catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Saito, J.; Suzuki, Y.; Fujita, T. Higher α-olefin polymerization behavior of a bis(phenoxy-imine)titanium complex/i-Bu3Al/Ph3CB(C6F5)4 catalyst system. Chem. Lett. 2003, 32, 236–237. [Google Scholar] [CrossRef]
- Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher a-olefins catalysed by aryloxo-modified half-titanocenes. RSC Adv. 2016, 6, 16203–16207. [Google Scholar] [CrossRef]
- Fries, A.; Mise, T.; Matsumoto, A.; Ohmori, H.; Wakatsuki, Y. Polymerization of hex-1-ene by homogeneous zirconocene and hafnocene catalysts in compressed solution. Chem. Commun. 1996, 783–784. [Google Scholar] [CrossRef] Synthesis of ultrahigh molecular weight poly(1-hexene) by (C5HMe4)2HfCl2–MAO catalyst under ultrahigh pressure (ex. 250 MPa).
- Segal, S.; Goldberg, I.; Kol, M. Zirconium and titanium diamine bis(phenolate) catalysts for α-olefin polymerization: From atactic oligo(1-hexene) to ultrahigh-molecular-weight isotactic poly(1-hexene). Organometallics 2005, 24, 200–202. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Enhancement of productivity, molecular weight and stereoregularity of 1-butene polymerization by MAO modification. Macromol. Chem. Phys. 2004, 205, 884–887. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Synthesis of ultra-high-molecular-weight poly(α-olefin)s by thiobis(phenoxy)titanium/modified methylaluminoxane system. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1107–1111. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Efficient living polymerization of 1-hexene by Cp*TiMe2(O-2,6-iPr2C6H3)-borate catalyst systems at low temperature. J. Mol. Catal. A Chem. 2004, 209, 9–17. [Google Scholar] [CrossRef]
- Jayaratne, K.C.; Sita, L.R. Stereospecific living Ziegler-Natta polymerization of 1-hexene. J. Am. Chem. Soc. 2000, 122, 958–959. [Google Scholar] [CrossRef]
- Domski, G.J.; Lobkovsky, E.B.; Coates, G.W. Polymerization of α-olefins with pridylamidohafnium ctalysts: Living bhavior and uexpected ioselectivity from a Cs-smmetric ctalyst pecursor. Macromolecules 2007, 40, 3510–3513. [Google Scholar] [CrossRef]
- Cai, Z.; Ohmagari, M.; Nakayama, Y.; Shiono, T. Highly active syndiospecific living polymerization of higher 1-alkene with ansa-fluorenylamidodimethyltitanium complex. Macromol. Rapid Commun. 2009, 30, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Kotzabasakis, V.; Mourmouris, S.; Pitsikalis, M.; Hadjichristidis, N.; Lohse, D.J. Synthesis and characterization of complex macromolecular architectures based on poly(α-olefins) utilizing a Cs-symmetry hafnium metallocene catalyst in combination with atom transfer radical polymerization (ATRP). Macromolecules 2011, 44, 1952–1968. [Google Scholar] [CrossRef]
- Kiesewetter, E.T.; Waymouth, R.M. Octahedral group IV bis(phenolate) catalysts for 1-hexene homopolymerization and ethylene/1-hexene copolymerization. Macromolecules 2013, 46, 2569–2575. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefinpolymerization and copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Nomura, K.; Fujita, K.; Fujiki, M. Effects of cyclopentadienyl fragment in ethylene, 1-hexene, and styrene polymerizations catalyzed by half-titanocenes containing ketimide ligand of the type, Cp’TiCl2(N=CtBu2). Catal. Commun. 2004, 5, 413–417. [Google Scholar] [CrossRef]
- Nomura, K.; Komatsu, T.; Imanishi, Y. Ligand effect in olefin polymerization catalyzed by (cyclopentadienyl)(aryloxy)titanium(IV) complexes, Cp’TiCl2(OAr)–MAO system. Ethylene/1-hexene copolymerization by (1,3-tBu2C5H3)TiCl2(O-2,6-iPr2C6H3)–MAO catalyst system. J. Mol. Catal. A Chem. 2000, 159, 127–137. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Syndiospecific styrene polymerization by (tert-BuC5H4)TiCl2(O-2,6-iPr2C6H3)–borate catalyst system. Catal. Commun. 2003, 4, 269–274. [Google Scholar] [CrossRef]
- Nomura, K.; Suzuki, N.; Kim, D.-H.; Kim, H.J. Effect of cocatalyst in ethylene/styrene copolymerization by aryloxo-modified half-titanocene–cocatalyst systems for exclusive synthesis of copolymers at high styrene concentrations. Macromol. React. Eng. 2012, 6, 351–356. [Google Scholar] [CrossRef]
- Nomura, K.; Pracha, S.; Phomphrai, K.; Katao, S.; Kim, D.-H.; Kim, H.J.; Suzuki, N. Synthesis and structural analysis of phenoxy-substituted half-titanocenes with different anionic ligands, Cp*TiX(Y)(O-2,6-iPr2C6H3): Effect of anionic ligands (X,Y) in ethylene/styrene copolymerization. J. Mol. Catal. A Chem. 2012, 365, 136–145. [Google Scholar] [CrossRef]
- Hagihara, H.; Shiono, T.; Ikeda, T. Living polymerization of propene and 1-hexene with the [t-BuNSiMe2Flu]TiMe2/B(C6F5)3 catalyst. Macromolecules 1998, 31, 3184–3188. [Google Scholar] [CrossRef]They proposed that Al(n-C8H17)3 interacts with the counteranion of the cationic active Ti species to improve coordinative unsaturation of the Ti species.
- Fukui, Y.; Murata, M.; Soga, K. Living polymerization of propylene and 1-hexene using bis-Cp type metallocene catalysts. Macromol. Rapid Commun. 1999, 20, 637–640. [Google Scholar] [CrossRef]
- Beckerle, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Living isospecific styrene polymerization by chiral benzyl titanium complexes that contain a tetradentate [OSSO]-type bis(phenolato) ligand. Organometallics 2006, 25, 3019–3026. [Google Scholar] [CrossRef]
- Asakura, T.; Demura, M.; Nishiyama, Y. Carbon-13 NMR spectral assignment of five polyolefins determined from the chemical shift calculation and the polymerization mechanism. Macromolecules 1991, 24, 2334–2340. [Google Scholar] [CrossRef]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl)(aryloxy)- titanium(IV) complexes-cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Catalyst | α-Olefin | Cocatalyst | Time/min | TON b | Activity c | Mnd | Mw/Mnd |
|---|---|---|---|---|---|---|---|
| Cp*TiCl2(O-2,6-iPr2C6H3) | OC | MAO | 30 | 9950 | 2240 | 501000 | 1.42 |
| Cp*TiCl2(O-2,6-iPr2C6H3) | DD | MAO | 30 | 4120 | 1390 | 525000 | 1.35 |
| Cp*TiMe2(O-2,6-iPr2C6H3) | OC | borate | 20 | 7080 | 2380 | 1970000 | 2.04 |
| Cp*TiMe2(O-2,6-iPr2C6H3) | DD | borate | 20 | 3730 | 1880 | 1320000 | 1.99 |
| [Me2Si(C5Me4)(NtBu)]TiCl2 | OC | MAO | 30 | 4810 | 1080 | 292000 | 1.64 |
| [Me2Si(C5Me4)(NtBu)]TiCl2 | DD | MAO | 30 | 2060 | 690 | 351000 | 1.33 |
| Cp2ZrCl2 | OC | MAO | 30 | 21300 | 4790 | 4100 | 1.55 |
| Cp2ZrCl2 | DD | MAO | 30 | 15400 | 5190 | 5000 | 1.57 |
| Run | Al(n-C8H17)3/AliBu3/Ti b | Temp./°C | Time/min | Polymer Yield c/mg | Activity d | TON e | Mnf × 10−4 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|
| 1 | -/500 | –30 | 5 | 926 | 11110 | 6600 | 40.1 | 1.52 |
| 10 | 1162 | 6970 | 8280 | 58.2 | 1.57 | |||
| 15 | 1644 | 6580 | 11700 | 68.8 | 1.61 | |||
| 20 | 1952 | 5860 | 13900 | 78.2 | 1.61 | |||
| 2 | 200/300 | −30 | 5 | 366 | 4390 | 2610 | 39.6 | 1.45 |
| 10 | 1130 | 6780 | 8050 | 61.3 | 1.43 | |||
| 15 | 1454 | 5820 | 10360 | 68.7 | 1.50 | |||
| 20 | 1708 | 5120 | 12170 | 76.4 | 1.52 | |||
| 3 | 400/100 | −30 | 5 | 348 | 4180 | 2480 | 40.8 | 1.32 |
| 10 | 752 | 4510 | 5360 | 58.0 | 1.36 | |||
| 15 | 1240 | 4960 | 8840 | 64.2 | 1.38 | |||
| 20 | 1372 | 4120 | 9780 | 70.4 | 1.37 | |||
| 4 | 400/100 | −40 | 10 | 250 | 1500 | 1780 | 31.9 | 1.26 |
| 15 | 408 | 1630 | 2910 | 39.0 | 1.30 | |||
| 20 | 602 | 1810 | 4290 | 47.7 | 1.25 | |||
| 30 | 976 | 1950 | 6960 | 56.9 | 1.25 | |||
| 45 | 1228 | 1640 | 8750 | 63.4 | 1.29 | |||
| 60 | 1620 | 1620 | 11500 | 66.8 | 1.29 | |||
| 5 | 300/200 | −50 | 15 | 250 | 1000 | 1780 | 36.2 | 1.10 |
| 30 | 490 | 980 | 3490 | 42.0 | 1.17 | |||
| 45 | 506 | 680 | 3610 | 43.1 | 1.21 | |||
| 60 | 534 | 530 | 3810 | 44.6 | 1.13 | |||
| 75 | 558 | 450 | 4000 | 45.0 | 1.13 | |||
| 90 | 582 | 390 | 4150 | 45.5 | 1.16 | |||
| 105 | 652 | 370 | 4650 | 46.1 | 1.14 | |||
| 120 | 684 | 340 | 4880 | 46.5 | 1.15 |
| Run | Al(n-C8H17)3/AliBu3/Ti b | Temp./°C | Time/min | Polymer Yield c/mg | Activity d | TON e | Mnf × 10−4 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|
| 6 | -/500 | –30 | 5 | 600 | 7200 | 3570 | 34.8 | 1.58 |
| 10 | 1236 | 7420 | 7340 | 48.0 | 1.66 | |||
| 15 | 2288 | 9150 | 13600 | 59.2 | 1.60 | |||
| 20 | 2982 | 8950 | 17700 | 67.4 | 1.68 | |||
| 7 | 200/300 | –30 | 5 | 4480 | 5380 | 2660 | 32.9 | 1.52 |
| 10 | 9300 | 5580 | 5530 | 41.2 | 1.53 | |||
| 15 | 1752 | 7010 | 10400 | 48.8 | 1.58 | |||
| 20 | 2094 | 6280 | 12400 | 55.4 | 1.61 | |||
| 8 | 400/100 | –30 | 5 | 398 | 4780 | 2370 | 36.1 | 1.56 |
| 10 | 668 | 4010 | 4000 | 45.4 | 1.52 | |||
| 15 | 1204 | 4820 | 7150 | 44.7 | 1.52 | |||
| 20 | 1674 | 5020 | 9950 | 48.4 | 1.54 | |||
| 9 | 400/100 | –40 | 10 | 304 | 1820 | 1810 | 35.3 | 1.25 |
| 15 | 428 | 1710 | 2540 | 39.3 | 1.22 | |||
| 20 | 574 | 1720 | 3410 | 40.9 | 1.25 | |||
| 30 | 654 | 1310 | 3890 | 42.7 | 1.26 | |||
| 45 | 682 | 910 | 4050 | 43.1 | 1.27 | |||
| 60 | 910 | 910 | 5410 | 45.2 | 1.29 | |||
| 10 | 250/250 | –50 | 60 | 267 | 530 | 3810 | 25.5 | 1.18 |
| 75 | 279 | 450 | 4000 | 31.2 | 1.17 | |||
| 90 | 291 | 390 | 4150 | 40.8 | 1.11 | |||
| 105 | 326 | 370 | 4650 | 48.1 | 1.18 | |||
| 120 | 342 | 340 | 4880 | 52.6 | 1.14 |
| Run | Al(n-C8H17)3/AliBu3/Ti b | Time/min | Polymer Yield c/mg | Activity d | TON e | Mnf × 10−4 | Mw/Mnf |
|---|---|---|---|---|---|---|---|
| 11 | -/500 | 5 | 376 | 4510 | 2680 | 31.7 | 1.34 |
| 10 | 480 | 2880 | 3420 | 34.1 | 1.37 | ||
| 20 | 602 | 1810 | 4290 | 43.0 | 1.32 | ||
| 30 | 944 | 1890 | 6730 | 52.7 | 1.35 | ||
| 45 | 1034 | 1380 | 7370 | 65.1 | 1.41 | ||
| 60 | 1734 | 1730 | 12400 | 74.8 | 1.41 | ||
| 75 | 2016 | 1610 | 14400 | 78.3 | 1.38 | ||
| 90 | 2396 | 1600 | 17100 | 82.2 | 1.43 | ||
| 105 | 2778 | 1580 | 19800 | 90.0 | 1.41 | ||
| 120 | 3132 | 1570 | 22300 | 101.6 | 1.38 | ||
| 12 | 200/300 | 20 | 202 | 610 | 1440 | 31.0 | 1.28 |
| 30 | 502 | 1000 | 3580 | 42.5 | 1.32 | ||
| 45 | 1000 | 1330 | 7130 | 53.5 | 1.32 | ||
| 60 | 1662 | 1660 | 11800 | 62.9 | 1.34 | ||
| 75 | 2232 | 1790 | 15900 | 73.4 | 1.28 | ||
| 90 | 2738 | 1850 | 19700 | 75.7 | 1.28 | ||
| 105 | 2980 | 1700 | 21200 | 82.5 | 1.31 | ||
| 120 | 3044 | 1520 | 21700 | 89.6 | 1.36 | ||
| 13 | 400/100 | 30 | 458 | 920 | 3260 | 30.6 | 1.25 |
| 45 | 702 | 940 | 5000 | 33.5 | 1.19 | ||
| 60 | 934 | 930 | 6660 | 38.1 | 1.23 | ||
| 75 | 1464 | 1170 | 10400 | 40.5 | 1.20 | ||
| 90 | 1750 | 1170 | 12500 | 47.3 | 1.23 | ||
| 105 | 2000 | 1140 | 14300 | 52.6 | 1.21 | ||
| 120 | 2292 | 1150 | 16300 | 59.7 | 1.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of Ultrahigh Molecular Weight Polymers with Low PDIs by Polymerizations of 1-Decene, 1-Dodecene, and 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Molecules 2019, 24, 1634. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081634
Nomura K, Pengoubol S, Apisuk W. Synthesis of Ultrahigh Molecular Weight Polymers with Low PDIs by Polymerizations of 1-Decene, 1-Dodecene, and 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Molecules. 2019; 24(8):1634. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081634
Chicago/Turabian StyleNomura, Kotohiro, Sarntamon Pengoubol, and Wannida Apisuk. 2019. "Synthesis of Ultrahigh Molecular Weight Polymers with Low PDIs by Polymerizations of 1-Decene, 1-Dodecene, and 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst" Molecules 24, no. 8: 1634. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081634