N-Heterocyclic Carbene Adducts of Alkynyl Functionalized 1,3,2-Dithioborolanes
Abstract
:1. Introduction
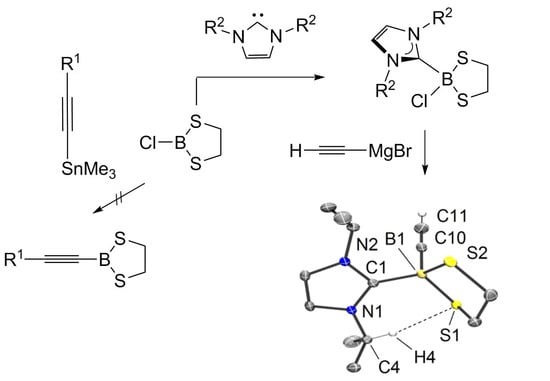
2. Results
3. Discussion
4. Materials and Methods
4.1. General Remarks
4.2. Analytical Methods
4.3. Synthetic Procedures
4.3.1. Compound 8a
4.3.2. Compound 8b
4.3.3. Compound 9a
4.3.4. Compound 9b
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sakakibara, R.; Sasaki, W.; Onda, Y.; Yamaguchi, M.; Ushirogochi, H.; Yuki, H.; Sato, K.; Nishio, M.; Egi, Y.; Takedomi, K.; et al. Discovery of Novel Pyrazole-Based Selective Aldosterone Synthase. (CYP11B2) Inhibitors: A New Template to Coordinate the Heme-Iron Motif of CYP11B2. J. Med. Chem. 2018, 61, 5594–5608. [Google Scholar] [CrossRef] [PubMed]
- Niermeier, P.; Blomeyer, S.; Bejaoui, Y.K.J.; Beckmann, J.L.; Neumann, B.; Stammler, H.-G.; Mitzel, N.W. Bidentate Boron Lewis Acids: Selectivity in Host-Guest Complex Formation. Angew. Chem. Int. Ed. 2019, 58, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Wiltshire, B.D.; Choi, P.; Savin, A.; Sun, S.; Mohammadpour, A.; Ferguson, M.J.; McDonald, R.; Farsinezhad, S.; Brown, A.; et al. Phosphorescence within Benzotellurophenes and Color Tunable Tellurophenes under Ambient Conditions. Chem. Commun. 2015, 51, 5444–5447. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; May, J.A. Enantioselective Organocatalytic Conjugate Addition of Organoboron Nucleophiles. Tetrahedron Lett. 2017, 58, 1535–1544. [Google Scholar] [CrossRef]
- Jouvin, K.; Evano, G. Beyond Ullmann-Goldberg Chemistry: Vinylation, Alkynylation, and Allenylation of Heteronucleophiles. In Copper-Mediated Cross-Coupling Reactions; Evano, G., Blanchard, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 187–238. [Google Scholar] [CrossRef]
- Jiao, J.; Nishihara, Y. Alkynylboron Compounds in Organic Synthesis. J. Organomet. Chem. 2012, 721–722, 3–16. [Google Scholar] [CrossRef]
- Kaufmann, D.E.; Ocal, N. Product Subclass 23: Alk-1-ynylboranes and Alkyn-1-ylboronates. Sci. Synth. 2004, 6, 635–658. [Google Scholar]
- Vaultier, M.; Alcaraz, G. Product Subclass 28: Vinylboranes. Sci. Synth. 2004, 6, 721–853. [Google Scholar]
- Zaidlewicz, M.; Krzeminski, M. Product Subclass 38: Trialkylboranes. Sci. Synth. 2004, 6, 1097–1215. [Google Scholar]
- Ishihara, K.; Gao, Q.; Yamamoto, H. Enantioselective Diels-Alder Reaction of α-Bromo-α,β-enals with Dienes under Catalysis by CAB. J. Org. Chem. 1993, 58, 6917–6919. [Google Scholar] [CrossRef]
- Hass, O.; Schierholt, A.; Jordan, M.; Luetzen, A. Improved Synthesis of Monohalogenated Cavitands and Their Use in the Synthesis of Further Functionalized Cavitands. Synthesis 2006, 2006, 519–527. [Google Scholar]
- Kinoshita, H.; Takahashi, H.; Miura, K. Regioselective Synthesis of Multisubstituted Benzenes by Palladium-Catalyzed Intermolecular Reaction of β-Iodo-β-silylstyrenes with Alkynes. Org. Lett. 2013, 15, 2962–2965. [Google Scholar] [CrossRef]
- Geny, A.; Leboeuf, D.; Rouquie, G.; Vollhardt, K.P.C.; Malacria, M.; Gandon, V.; Aubert, C. Cobalt(I)-Mediated Preparation of Polyborylated Cyclohexadienes: Scope, Limitations, and Mechanistic Insight. Chem. Eur. J. 2007, 13, 5408–5425. [Google Scholar] [CrossRef]
- Hu, J.-R.; Liu, L.-H.; Hu, X.; Ye, H.-D. Ag (I)-catalyzed C–H borylation of terminal alkynes. Tetrahedron 2014, 70, 5815–5819. [Google Scholar] [CrossRef]
- Deloux, L.; Skrzypczak-Jankun, E.; Cheesman, B.V.; Srebnik, M.; Sabat, M. First Example of STable 1,1-Bimetalloalkenes of Boron and Zirconium: Synthesis, Reactivity. X-ray Analysis, and NMR Studies. J. Am. Chem. Soc. 1994, 116, 10302–10303. [Google Scholar] [CrossRef]
- Romero, E.A.; Jazzar, R.; Bertrand, G. Copper-Catalyzed Dehydrogenative Borylation of Terminal Alkynes with Pinacolborane. Chem. Sci. 2017, 8, 165–168. [Google Scholar] [CrossRef]
- Lee, C.I.; DeMott, J.C.; Pell, C.J.; Christopher, A.; Zhou, J.; Bhuvanesh, N.; Ozerov, O.V. Ligand Survey Results in Identification of PNP Pincer Complexes of Iridium as Long-Lived and Chemoselective Catalysts for Dehydrogenative Borylation of Terminal Alkynes. Chem. Sci. 2015, 6, 6572–6582. [Google Scholar] [CrossRef]
- Nykaza, T.V.; Cooper, J.C.; Li, G.; Mahieu, N.; Ramirez, A.; Luzung, M.R.; Radosevich, A.T. Intermolecular Reductive C−N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc. 2018, 140, 15200–15205. [Google Scholar] [CrossRef]
- Wei, D.; Carboni, B.; Sortais, J.-B.; Darcel, C. Iron-Catalyzed Dehydrogenative Borylation of Terminal Alkynes. Adv. Synth. Catal. 2018, 360, 3649–3654. [Google Scholar] [CrossRef]
- Gu, Y.; Pritzkow, H.; Siebert, W. Synthesis and Reactivity of Monoborylacetylene Derivatives. Eur. J. Inorg. Chem. 2001, 373–379. [Google Scholar] [CrossRef]
- Lawson, J.R.; Clark, E.R.; Cade, I.A.; Solomon, S.A.; Ingleson, M.J. Haloboration of Internal Alkynes with Boronium and Borenium Cations as a Route to Tetrasubstituted Alkenes. Angew. Chem. Int. Ed. 2013, 52, 7518–7522. [Google Scholar] [CrossRef]
- Yao, H.; Sabat, M.; Grimes, R.N.; Zanello, P.; Fabrizi de Biani, F. Alkynyl-Linked Poly(cobaltacarboranes). Directed Synthesis, Structures, and Electrochemistry of Linear Complexes and Benzene-Centered Pinwheels. Organometallics 2003, 22, 2581–2593. [Google Scholar] [CrossRef]
- Gomez-Bengoa, E.; Helm, M.D.; Plant, A.; Harrity, J.P.A. The Participation of Alkynylboronates in Inverse Electron Demand [4 + 2] Cycloadditions: A Mechanistic Study. J. Am. Chem. Soc. 2007, 129, 2691–2699. [Google Scholar] [CrossRef]
- Jiang, Y.; Diagne, A.B.; Thomson, R.J.; Schaus, S.E. Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions. J. Am. Chem. Soc. 2017, 139, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Böser, R.; Haufe, L.C.; Freytag, M.; Jones, P.G.; Hörner, G.; Frank, R. Completing the Series of Boron -Nucleophilic Cyanoborates: Boryl Anions of Type NHC–B(CN)2−. Chem. Sci. 2017, 8, 6274–6280. [Google Scholar] [CrossRef]
- Goswami, A.; Pritzkow, H.; Rominger, F.; Siebert, W. Synthesis, Structures and Reactivity of Mono- and Diborylacetylenes. Eur. J. Inorg. Chem. 2004, 2004, 4223–4231. [Google Scholar] [CrossRef]
- Finch, A.; Pearn, J. Preparation and Stability of Boron Heterocycles Containing Sulfur. Tetrahedron 1964, 20, 173–176. [Google Scholar] [CrossRef]
- Solovyev, A.; Chu, Q.; Geib, S.J.; Fensterbank, L.; Malacria, M.; Lacote, E.; Curran, D.P. Substitution Reactions at Tetracoordinate Boron: Synthesis of N-Heterocyclic Carbene Boranes with Boron-Heteroatom Bonds. J. Am. Chem. Soc. 2010, 132, 15072–15080. [Google Scholar] [CrossRef]
- Silva Valverde, M.F.; Schweyen, P.; Gisinger, D.; Bannenberg, T.; Freytag, M.; Kleeberg, C.; Tamm, M. N-Heterocyclic Carbene Stabilized Boryl Radicals. Angew. Chem. Int. Ed. 2017, 56, 1135–1140. [Google Scholar] [CrossRef]
- Farrell, J.M.; Posaratnanathan, R.T.; Stephan, D.W. A Family of N-Heterocyclic Carbene-Stabilized Borenium Ions for Metal-Free Imine Hydrogenation Catalysis. Chem. Sci. 2015, 6, 2010–2015. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. N-Heterocyclic Carbenes. In N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Nolan, S.P., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–24. [Google Scholar]
- Schaub, T.; Backes, M.; Radius, U. Nickel(0) Complexes of N-Alkyl-Substituted N-Heterocyclic Carbenes and Their Use in the Catalytic Carbon−Carbon Bond Activation of Biphenylene. Organometallics 2006, 25, 4196–4206. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Krafczyk, R.; Schmutzler, R. Imidazolylidenes, Imidazolinylidenes and Imidazolidines. Tetrahedron 1999, 55, 14523–14534. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böser, R.; Denker, L.; Frank, R. N-Heterocyclic Carbene Adducts of Alkynyl Functionalized 1,3,2-Dithioborolanes. Molecules 2019, 24, 1690. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091690
Böser R, Denker L, Frank R. N-Heterocyclic Carbene Adducts of Alkynyl Functionalized 1,3,2-Dithioborolanes. Molecules. 2019; 24(9):1690. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091690
Chicago/Turabian StyleBöser, Richard, Lars Denker, and René Frank. 2019. "N-Heterocyclic Carbene Adducts of Alkynyl Functionalized 1,3,2-Dithioborolanes" Molecules 24, no. 9: 1690. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091690