Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips
Abstract
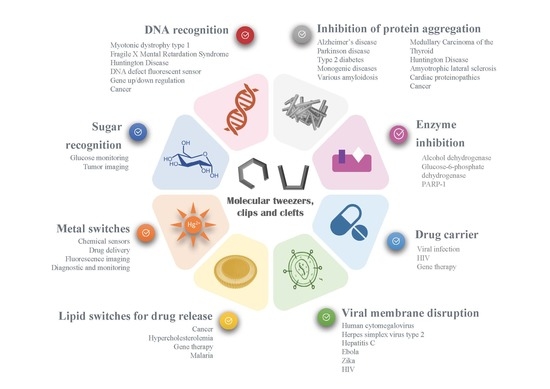
:1. Introduction to Molecular Tweezers, Clefts and Clips
2. Recognition of Biological Guests
2.1. Nucleic Acid Recognition
2.1.1. Purine Bases Recognition
2.1.2. Trinucleotide Repeat Recognition in Myotonic Dystrophy Type 1 (DM1)
2.1.3. Mismatch Recognition
2.1.4. Trinucleotide repeats in Fragile X Mental Retardation Syndrome and Huntington disease
2.1.5. DNA Binding and Anticancer Agents
2.2. Amino Acids and Protein Recognition
2.2.1. Enzyme Inhibition
2.2.2. Prevention of Protein Aggregation In Amyloidosis
2.2.3. Modulation of Protein-Protein Interaction (PPI)
2.2.4. Inhibition of Enveloped Viruses
2.3. Sugar Recognition
3. Stimuli-Responsive Tweezers for Drug Delivery
3.1. Metal Switches for Sensing or Delivery
3.2. Lipid Switches for Controlled Drug Release
4. Summary and Outlook
4.1. How to Use Molecular Tweezers?
- (i)
- A new drug candidate. In this category, CLR01 is the most advanced compound, showing multiple applications and preclinical data. To progress in the drug development process, the drug-like properties need to be established (absorption, distribution, pharmacokinetics, metabolism, elimination) and might represent a challenge due to the generally hydrophobic aromatic structure.
- (ii)
- A biosensor, for the detection of various molecules or supramolecular assemblies, from cations to DNA mismatches. In this field, the highest challenge is the selectivity and sensitivity of the system in biological medium.
- (iii)
- A drug delivery system, usually with responsive properties. The conformational change offers a faster kinetics and a better controlled assembly over other responsive systems, such as hydrolysable polymers. Nevertheless, the biocompatibility and stability of such new excipients with biological media need to be investigated.
4.2. In Which Disease Could Molecular Tweezers be Used?
4.3. What are the Next Steps for Pharmaceutical Development of Molecular Tweezers?
4.4. Towards New Molecular Tweezers?
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bier, D.; Rose, R.; Bravo-Rodriguez, K.; Bartel, M.; Ramirez-Anguita, J.M.; Dutt, S.; Wilch, C.; Klärner, F.-G.; Sanchez-Garcia, E.; Schrader, T.; et al. Molecular tweezers modulate 14-3-3 protein–protein interactions. Nat. Chem. 2013, 5, 234–239. [Google Scholar] [CrossRef]
- Zhou, X.; Pathak, P.; Jayawickramarajah, J. Design, synthesis, and applications of DNA-macrocyclic host conjugates. Chem. Commun. 2018, 54, 11668–11680. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.L.; Fan, Y.S.; Wang, H. Host-guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv. Mat. 2013, 25, 3888–3898. [Google Scholar] [CrossRef]
- Feng, W.; Jin, M.; Yang, K.; Pei, Y.; Pei, Z. Supramolecular delivery systems based on pillararenes. Chem. Commun. 2018, 54, 13626–13640. [Google Scholar] [CrossRef]
- Leblond, J.; Petitjean, A. Molecular tweezers: Concepts and applications. Chem. Phys. Chem. 2011, 12, 1043–1051. [Google Scholar] [CrossRef]
- Hardouin-Lerouge, M.; Hudhomme, P.; Salle, M. Molecular clips and tweezers hosting neutral guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Whitlock, H.W. Molecular tweezers: A simple model of bifunctional intercalation. J. Am. Chem. Soc. 1978, 100, 4921–4922. [Google Scholar] [CrossRef]
- Rebek, J.; Askew, B.; Islam, N.; Killoran, M.; Nemeth, D.; Wolak, R. Synthetic receptors: Size and shape recognition within a molecular cleft. J. Am. Chem. Soc. 1985, 107, 6736–6738. [Google Scholar] [CrossRef]
- Harmata, M. Molecular Clefts and Tweezers. In Encyclopedia of Supramolecular Chemistry; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Rebek, J.; Askew, B.; Killoran, M.; Nemeth, D.; Lin, F.T. Convergent functional groups. 3. A molecular cleft recognizes substrates of complementary size, shape, and functionality. J. Am. Chem. Soc. 1987, 109, 2426–2431. [Google Scholar] [CrossRef]
- Rebek, J.; Askew, B.; Nemeth, D.; Parris, K. Convergent functional groups. 4. Recognition and transport of amino acids across a liquid membrane. J. Am. Chem. Soc. 1987, 109, 2432–2434. [Google Scholar] [CrossRef]
- Askew, B.; Ballester, P.; Buhr, C.; Jeong, K.S.; Jones, S.; Parris, K.; Williams, K.; Rebek, J. Molecular recognition with convergent functional groups. VI. Synthetic and structural studies with a model receptor for nucleic acid components. J. Am. Chem. Soc. 1989, 111, 1082–1090. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Schrader, T. Aromatic Interactions by Molecular Tweezers and Clips in Chemical and Biological Systems. Acc. Chem. Res. 2013, 46, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L. In Control of Motion: From Molecular Switches to Molecular Motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef]
- Zimmerman, S.C. A journey in bioinspired supramolecular chemistry: From molecular tweezers to small molecules that target myotonic dystrophy. Beilstein J. Org. Chem. 2016, 12, 125–138. [Google Scholar] [CrossRef]
- Schrader, T.; Bitan, G.; Klarner, F.G. Molecular tweezers for lysine and arginine—Powerful inhibitors of pathologic protein aggregation. Chem. Commun. 2016, 52, 11318–11334. [Google Scholar] [CrossRef] [PubMed]
- Harmata, M. Chiral Molecular Tweezers. Acc. Chem. Res. 2004, 37, 862–873. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Askew, B.; Ballester, P.; Buhr, C.; Jeong, K.S.; Jones, S.; Rebek, J. Molecular recognition with convergent functional groups. VII. Energetics of adenine binding with model receptors. J. Am. Chem. Soc. 1989, 111, 1090–1094. [Google Scholar] [CrossRef]
- Castellano, R.K.; Gramlich, V.; Diederich, F. Rebek Imides and Their Adenine Complexes: Preferences for Hoogsteen Binding in the Solid State and in Solution. Chem. Eur. J. 2002, 8, 118–129. [Google Scholar] [CrossRef]
- Faraoni, R.; Blanzat, M.; Kubicek, S.; Braun, C.; Schweizer, W.B.; Gramlich, V.; Diederich, F. New Rebek imide-type receptors for adenine featuring acetylene-linked [small pi]-stacking platforms. Org. Biomol. Chem. 2004, 2, 1962–1964. [Google Scholar] [CrossRef]
- Park, T.K.; Schroeder, J.; Rebek, J. Convergent functional groups XI. Selective binding of guanosine derivatives. Tetrahedron 1991, 47, 2507–2518. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; da Silva, E.J.T.; Pereira, J.A.C.; Ortuso, F.; Alcaro, S.; Pinto, M.M.M. Molecular clefts of Rebek revisited: Potential application as drug carriers for the antiviral acyclovir. J. Inclusion Phenom. Macrocyclic Chem. 2015, 83, 203–208. [Google Scholar] [CrossRef]
- Plante, J.P.; Glass, T.E. Shape-Selective Fluorescent Sensing Ensemble Using a Tweezer-Type Metalloreceptor. Org. Lett. 2006, 8, 2163–2166. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; VanZyl, C.M. Rigid molecular tweezers: Synthesis, characterization, and complexation chemistry of a diacridine. J. Am. Chem. Soc. 1987, 109, 7894–7896. [Google Scholar] [CrossRef]
- Zimmerman, S.C.; Wu, W.; Zeng, Z. Complexation of nucleotide bases by molecular tweezers with active site carboxylic acids: effects of microenvironment. J. Am. Chem. Soc. 1991, 113, 196–201. [Google Scholar] [CrossRef]
- Arambula, J.F.; Ramisetty, S.R.; Baranger, A.M.; Zimmerman, S.C. A simple ligand that selectively targets CUG trinucleotide repeats and inhibits MBNL protein binding. Proc. Natl. Acad. Sci. USA 2009, 106, 16068–16073. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, A.H.; Nguyen, L.; Fu, Y.; Miller, K.A.; Baranger, A.M.; Zimmerman, S.C. A Novel CUGexp·MBNL1 Inhibitor with Therapeutic Potential for Myotonic Dystrophy Type 1. ACS Chem. Biol. 2013, 8, 1037–1043. [Google Scholar] [CrossRef]
- Jahromi, A.H.; Fu, Y.; Miller, K.A.; Nguyen, L.; Luu, L.M.; Baranger, A.M.; Zimmerman, S.C. Developing Bivalent Ligands to Target CUG Triplet Repeats, the Causative Agent of Myotonic Dystrophy Type 1. J. Med. Chem. 2013, 56, 9471–9481. [Google Scholar] [CrossRef]
- Wong, C.H.; Nguyen, L.; Peh, J.; Luu, L.M.; Sanchez, J.S.; Richardson, S.L.; Tuccinardi, T.; Tsoi, H.; Chan, W.Y.; Chan, H.Y.; et al. Targeting toxic RNAs that cause myotonic dystrophy type 1 (DM1) with a bisamidinium inhibitor. J. Am. Chem. Soc. 2014, 136, 6355–6361. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.M.; Nguyen, L.; Peng, S.; Lee, J.; Lee, H.Y.; Wong, C.-H.; Hergenrother, P.J.; Chan, H.Y.E.; Zimmerman, S.C. A Potent Inhibitor of Protein Sequestration by Expanded Triplet (CUG) Repeats that Shows Phenotypic Improvements in a Drosophila Model of Myotonic Dystrophy. ChemMedChem 2016, 11, 1428–1435. [Google Scholar] [CrossRef]
- Nguyen, L.; Luu, L.M.; Peng, S.; Serrano, J.F.; Chan, H.Y.; Zimmerman, S.C. Rationally designed small molecules that target both the DNA and RNA causing myotonic dystrophy type 1. J. Am. Chem. Soc. 2015, 137, 14180–14189. [Google Scholar] [CrossRef]
- Nakatani, K.; Sando, S.; Kumasawa, H.; Kikuchi, J.; Saito, I. Recognition of Guanine−Guanine Mismatches by the Dimeric Form of 2-Amino-1,8-naphthyridine. J. Am. Chem. Soc. 2001, 123, 12650–12657. [Google Scholar] [CrossRef]
- Nakatani, K.; Sando, S.; Saito, I. Scanning of guanine–guanine mismatches in DNA by synthetic ligands using surface plasmon resonance. Nat. Biotechnol. 2001, 19, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Kobori, A.; Kumasawa, H.; Saito, I. Highly sensitive detection of GG mismatched DNA by surfaces immobilized naphthyridine dimer through poly(ethylene oxide) linkers. Bioorg. Med. Chem. Lett. 2004, 14, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Kobori, A.; Horie, S.; Suda, H.; Saito, I.; Nakatani, K. The SPR Sensor Detecting Cytosine−Cytosine Mismatches. J. Am. Chem. Soc. 2004, 126, 557–562. [Google Scholar] [CrossRef]
- Nakatani, K.; Toda, M.; He, H. A dimeric form of N-methoxycarbonyl-2-amino-1,8-naphthyridine bound to the A-A mismatch in the CAG/CAG base triad in dsRNA. Bioorg. Med. Chem. Lett. 2013, 23, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Dohno, C.; Nakatani, K. Bidirectional Control of Gold Nanoparticle Assembly by Turning On and Off DNA Hybridization with Thermally Degradable Molecular Glue. Chem. Biol. Chem. 2007, 8, 483–485. [Google Scholar] [CrossRef]
- Dohno, C.; Uno, S.-N.; Nakatani, K. Photoswitchable Molecular Glue for DNA. J. Am. Chem. Soc. 2007, 129, 11898–11899. [Google Scholar] [CrossRef]
- Kumar Verma, R.; Takei, F.; Nakatani, K. Synthesis and Photophysical Properties of Fluorescence Molecular Probe for Turn-ON-Type Detection of Cytosine Bulge DNA. Org. Lett. 2016, 18, 3170–3173. [Google Scholar] [CrossRef]
- Shibata, T.; Nakatani, K. Bicyclic and tricyclic C-C mismatch-binding ligands bind to CCG trinucleotide repeat DNAs. Chem. Commun. 2018, 54, 7074–7077. [Google Scholar] [CrossRef]
- Hagihara, M.; He, H.; Kimura, M.; Nakatani, K. A small molecule regulates hairpin structures in d(CGG) trinucleotide repeats. Bioorg. Med. Chem. Lett. 2012, 22, 2000–2003. [Google Scholar] [CrossRef]
- He, H.; Xia, J.; Peng, X.; Chang, G.; Zhang, X.; Wang, Y.; Nakatani, K.; Lou, Z.; Wang, S. Facile electrochemical biosensor based on a new bifunctional probe for label-free detection of CGG trinucleotide repeat. Biosens. Bioelectron. 2013, 49, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; He, H.; Nakatani, K. Small molecule modulates hairpin structures in CAG trinucleotide repeats. Chembiochem 2011, 12, 1686–1689. [Google Scholar] [CrossRef]
- Yamada, T.; Miki, S.; Ul’ Husna, A.; Michikawa, A.; Nakatani, K. Synthesis of Naphthyridine Carbamate Dimer (NCD) Derivatives Modified with Alkanethiol and Binding Properties of G-G Mismatch DNA. Org. Lett. 2017, 19, 4163–4166. [Google Scholar] [CrossRef]
- Dohno, C.; Kohyama, I.; Kimura, M.; Hagihara, M.; Nakatani, K. A Synthetic Riboswitch that Operates using a Rationally Designed Ligand–RNA Pair. Angew. Chem. Int. Ed. 2013, 52, 9976–9979. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Iida, K.; Murata, A.; Denawa, M.; Hagiwara, M.; Nakatani, K. Synthetic ligand promotes gene expression by affecting GC sequence in promoter. Bioorg. Med. Chem. Lett. 2017, 27, 3391–3394. [Google Scholar] [CrossRef]
- Bousquet, P.F.; Braña, M.F.; Conlon, D.; Fitzgerald, K.M.; Perron, D.; Cocchiaro, C.; Miller, R.; Moran, M.; George, J.; Qian, X.-D.; et al. Preclinical Evaluation of LU 79553: A Novel Bis-naphthalimide with Potent Antitumor Activity. Cancer Res. 1995, 55, 1176–1180. [Google Scholar]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef]
- Van Vliet, L.D.; Ellis, T.; Foley, P.J.; Liu, L.; Pfeffer, F.M.; Russell, R.A.; Warrener, R.N.; Hollfelder, F.; Waring, M.J. Molecular Recognition of DNA by Rigid [n]-Polynorbornane-Derived Bifunctional Intercalators: Synthesis and Evaluation of Their Binding Properties. J. Med. Chem. 2007, 50, 2326–2340. [Google Scholar] [CrossRef]
- Fokkens, M.; Jasper, C.; Schrader, T.; Koziol, F.; Ochsenfeld, C.; Polkowska, J.; Lobert, M.; Kahlert, B.; Klärner, F.-G. Selective Complexation of N-Alkylpyridinium Salts: Binding of NAD+ in Water. Chem. Eur. J. 2004, 11, 477–494. [Google Scholar] [CrossRef]
- Fokkens, M.; Schrader, T.; Klärner, F.-G. A Molecular Tweezer for Lysine and Arginine. J. Am. Chem. Soc. 2005, 127, 14415–14421. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Talbiersky, P.; Polkowska, J.; Bastkowski, F.; Schaller, T.; de Groot, H.; Klärner, F.G.; Schrader, T. A Mechanism of Efficient G6PD Inhibition by a Molecular Clip. Angew. Chem. Int. Ed. 2009, 48, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Wilch, C.; Talbiersky, P.; Berchner-Pfannschmidt, U.; Schaller, T.; Kirsch, M.; Klärner, F.G.; Schrader, T. Molecular Tweezers Inhibit PARP-1 by a New Mechanism. Eur. J. Org. Chem. 2017, 2017, 2223–2229. [Google Scholar] [CrossRef]
- Talbiersky, P.; Bastkowski, F.; Klärner, F.-G.; Schrader, T. Molecular Clip and Tweezer Introduce New Mechanisms of Enzyme Inhibition. J. Am. Chem. Soc. 2008, 130, 9824–9828. [Google Scholar] [CrossRef]
- Sinha, S.; Lopes, D.H.; Du, Z.; Pang, E.S.; Shanmugam, A.; Lomakin, A.; Talbiersky, P.; Tennstaedt, A.; McDaniel, K.; Bakshi, R.; et al. Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. J. Am. Chem. Soc. 2011, 133, 16958–16969. [Google Scholar] [CrossRef]
- Attar, A.; Ripoli, C.; Riccardi, E.; Maiti, P.; Li Puma, D.D.; Liu, T.; Hayes, J.; Jones, M.R.; Lichti-Kaiser, K.; Yang, F.; et al. Protection of primary neurons and mouse brain from Alzheimer’s pathology by molecular tweezers. Brain 2012, 135, 3735–3748. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Chan, W.-T.C.; Klärner, F.-G.; Schrader, T.; Bitan, G. Safety and pharmacological characterization of the molecular tweezer CLR01—A broad-spectrum inhibitor of amyloid proteins’ toxicity. BMC Pharmacol. Toxicol. 2014, 15. [Google Scholar] [CrossRef]
- Acharya, S.; Safaie, B.M.; Wongkongkathep, P.; Ivanova, M.I.; Attar, A.; Klarner, F.G.; Schrader, T.; Loo, J.A.; Bitan, G.; Lapidus, L.J. Molecular basis for preventing alpha-synuclein aggregation by a molecular tweezer. J. Biol. Chem. 2014, 289, 10727–10737. [Google Scholar] [CrossRef]
- Prabhudesai, S.; Sinha, S.; Attar, A.; Kotagiri, A.; Fitzmaurice, A.G.; Lakshmanan, R.; Ivanova, M.I.; Loo, J.A.; Klärner, F.-G.; Schrader, T.; et al. A Novel “Molecular Tweezer” Inhibitor of α-Synuclein Neurotoxicity in Vitro and in Vivo. Neurotherapeutics 2012, 9, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Subramaniam, S.R.; Magen, I.; Lee, P.; Hayes, J.; Attar, A.; Zhu, C.; Franich, N.R.; Bove, N.; De La Rosa, K.; et al. A Molecular Tweezer Ameliorates Motor Deficits in Mice Overexpressing α-Synuclein. Neurotherapeutics 2017, 14, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Pereira-Henriques, A.; Attar, A.; Klarner, F.G.; Schrader, T.; Bitan, G.; Gales, L.; Saraiva, M.J.; Almeida, M.R. Molecular tweezers targeting transthyretin amyloidosis. Neurotherapeutics 2014, 11, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.H.; Attar, A.; Nair, G.; Hayden, E.Y.; Du, Z.; McDaniel, K.; Dutt, S.; Bravo-Rodriguez, K.; Mittal, S.; Klarner, F.G.; et al. Molecular tweezers inhibit islet amyloid polypeptide assembly and toxicity by a new mechanism. ACS Chem. Biol. 2015, 10, 1555–1569. [Google Scholar] [CrossRef]
- Herzog, G.; Shmueli, M.D.; Levy, L.; Engel, L.; Gazit, E.; Klärner, F.-G.; Schrader, T.; Bitan, G.; Segal, D. The Lys-Specific Molecular Tweezer, CLR01, Modulates Aggregation of the Mutant p53 DNA Binding Domain and Inhibits Its Toxicity. Biochemistry 2015, 54, 3729–3738. [Google Scholar] [CrossRef]
- Xu, N.; Bitan, G.; Schrader, T.; Klarner, F.G.; Osinska, H.; Robbins, J. Inhibition of Mutant alphaB Crystallin-Induced Protein Aggregation by a Molecular Tweezer. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Meng, H.; Wongkongkathep, P.; Corrales, C.I.; Sepanj, N.; Atlasi, R.S.; Klärner, F.-G.; Schrader, T.; Spencer, M.J.; Loo, J.A.; et al. The molecular tweezer CLR01 inhibits aberrant superoxide dismutase 1 (SOD1) self-assembly in vitro and in the G93A-SOD1 mouse model of ALS. J. Biol. Chem. 2019, 294, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Vopel, T.; Bravo-Rodriguez, K.; Mittal, S.; Vachharajani, S.; Gnutt, D.; Sharma, A.; Steinhof, A.; Fatoba, O.; Ellrichmann, G.; Nshanian, M.; et al. Inhibition of Huntingtin Exon-1 Aggregation by the Molecular Tweezer CLR01. J. Am. Chem. Soc. 2017, 139, 5640–5643. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Koon, A.C.; Chen, Z.S.; Wei, Y.; An, Y.; Li, W.; Lau, M.H.Y.; Lau, K.-F.; Ngo, J.C.K.; Wong, C.-H.; et al. AQAMAN, a bisamidine-based inhibitor of toxic protein inclusions in neurons, ameliorates cytotoxicity in polyglutamine disease models. J. Biol. Chem. 2018, 294, 2757–2770. [Google Scholar] [CrossRef]
- Bier, D.; Mittal, S.; Bravo-Rodriguez, K.; Sowislok, A.; Guillory, X.; Briels, J.; Heid, C.; Bartel, M.; Wettig, B.; Brunsveld, L.; et al. The Molecular Tweezer CLR01 Stabilizes a Disordered Protein-Protein Interface. J. Am. Chem. Soc. 2017, 139, 16256–16263. [Google Scholar] [CrossRef]
- Lump, E.; Castellano, L.M.; Meier, C.; Seeliger, J.; Erwin, N.; Sperlich, B.; Sturzel, C.M.; Usmani, S.; Hammond, R.M.; von Einem, J.; et al. A molecular tweezer antagonizes seminal amyloids and HIV infection. eLife 2015, 4. [Google Scholar] [CrossRef]
- Röcker, A.E.; Müller, J.A.; Dietzel, E.; Harms, M.; Krüger, F.; Heid, C.; Sowislok, A.; Riber, C.F.; Kupke, A.; Lippold, S.; et al. The molecular tweezer CLR01 inhibits Ebola and Zika virus infection. Antiviral Res. 2018, 152, 26–35. [Google Scholar] [CrossRef]
- Miron, C.E.; Petitjean, A. Sugar recognition: Designing artificial receptors for applications in biological diagnostics and imaging. ChemBioChem 2015, 16, 365–379. [Google Scholar] [CrossRef]
- Benito, J.M.; Gómez-García, M.; Jiménez Blanco, J.L.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate-Based Receptors with Multiple Thiourea Binding Sites. Multipoint Hydrogen Bond Recognition of Dicarboxylates and Monosaccharides. J. Org. Chem. 2001, 66, 1366–1372. [Google Scholar] [CrossRef]
- James, T.D.; Samankumara, S.K.R.A.; Seiji, S. A Glucose-Selective Molecular Fluorescence Sensor. Angew. Chem. Int. Ed. 1994, 33, 2207–2209. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.R.A.S.; Iguchi, R.; Shinkai, S. Novel Saccharide-Photoinduced Electron Transfer Sensors Based on the Interaction of Boronic Acid and Amine. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, M.; Fan, H.; Yang, W.; Ni, W.; Karnati, V.V.R.; Gao, S.; Carson, J.; Weston, B.; Wang, B. A fluorescent bisboronic acid compound that selectively labels cells expressing oligosaccharide Lewis X. Bioorg. Med. Chem. Lett. 2015, 25, 2501–2504. [Google Scholar] [CrossRef]
- Wang, Y.E.; Rong, R.; Chen, H.; Zhu, M.; Wang, B.; Li, X. Triazole-linked fluorescent bisboronic acid capable of selective recognition of the Lewis Y antigen. Bioorg. Med. Chem. Lett. 2017, 27, 1983–1988. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, D.; Wang, K.; Liu, Z.; Weston, B.; Wang, B. Fluorescent conjugate of sLex-selective bisboronic acid for imaging application. Bioorg. Med. Chem. Lett. 2013, 23, 6307–6309. [Google Scholar] [CrossRef]
- Jia, S.; Fong, W.-K.; Graham, B.; Boyd, B.J. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem. Mater. 2018, 30, 2873–2887. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef]
- Petitjean, A.; Khoury, R.G.; Kyritsakas, N.; Lehn, J.-M. Dynamic Devices. Shape Switching and Substrate Binding in Ion-Controlled Nanomechanical Molecular Tweezers. J. Am. Chem. Soc. 2004, 126, 6637–6647. [Google Scholar] [CrossRef]
- Ulrich, S.; Petitjean, A.; Lehn, J.-M. Metallo-Controlled Dynamic Molecular Tweezers: Design, Synthesis, and Self-Assembly by Metal-Ion Coordination. Eur. J. Inorg. Chem. 2010, 2010, 1913–1928. [Google Scholar] [CrossRef]
- Doistau, B.; Tron, A.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Gontard, G.; Marvaud, V.; Hasenknopf, B.; Vives, G. Terpy(Pt-salphen)2 switchable luminescent molecular tweezers. Chem. Eur. J. 2014, 20, 15799–15807. [Google Scholar] [CrossRef]
- Doistau, B.; Rossi-Gendron, C.; Tron, A.; McClenaghan, N.D.; Chamoreau, L.M.; Hasenknopf, B.; Vives, G. Switchable platinum-based tweezers with Pt-Pt bonding and selective luminescence quenching. Dalton Trans. 2015, 44, 8543–8551. [Google Scholar] [CrossRef] [PubMed]
- Benda, L.; Doistau, B.; Hasenknopf, B.; Vives, G. Synthesis and Guest Recognition of Switchable Pt-Salphen Based Molecular Tweezers. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Doistau, B.; Benda, L.; Cantin, J.-L.; Chamoreau, L.-M.; Ruiz, E.; Marvaud, V.; Hasenknopf, B.; Vives, G. Six States Switching of Redox-Active Molecular Tweezers by Three Orthogonal Stimuli. J. Am. Chem. Soc. 2017, 139, 9213–9220. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Han, Y.; Ao, L.; Wang, F. Bis[alkynylplatinum(II)] Terpyridine Molecular Tweezer/Guest Recognition Enhanced by Intermolecular Hydrogen Bonds: Phototriggered Complexation via the “Caging” Strategy. Organometallics 2016, 35, 2850–2853. [Google Scholar] [CrossRef]
- Lou, J.; Zhang, X.; Best, M.D. Lipid Switches: Stimuli-Responsive Liposomes through Conformational Isomerism Driven by Molecular Recognition. Chem. Eur. J. 2019, 25, 20–25. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Brazdova, B.; Zhang, N.; Samoshin, V.V.; Guo, X. Trans-2-Aminocyclohexanol as a pH-sensitive conformational switch in lipid amphiphiles. Chem. Commun. 2008, 39, 4774–4776. [Google Scholar] [CrossRef] [PubMed]
- Samoshina, N.M.; Liu, X.; Brazdova, B.; Franz, A.H.; Samoshin, V.V.; Guo, X. Fliposomes: pH-Sensitive Liposomes Containing a trans-2-morpholinocyclohexanol-Based Lipid That Performs a Conformational Flip and Triggers an Instant Cargo Release in Acidic Medium. Pharmaceutics 2011, 3, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Samoshina, N.M.; Samoshin, V.V.; Franz, A.H.; Guo, X. Fliposomes: trans-2-aminocyclohexanol-based amphiphiles as pH-sensitive conformational switches of liposome membrane—A structure-activity relationship study. Chem. Phys. Lipids 2018, 210, 129–141. [Google Scholar] [CrossRef]
- Samoshin, V.V. Fliposomes: stimuli-triggered conformational flip of novel amphiphiles causes an instant cargo release from liposomes. Biomol. Concepts 2014, 5, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Samoshina, N.M.; Samoshin, V.V.; Franz, A.H.; Guo, X. Trans-2-Aminocyclohexanol-based amphiphiles as highly efficient helper lipids for gene delivery by lipoplexes. Biochim. Biophys. Acta 2015, 1848, 3113–3125. [Google Scholar] [CrossRef]
- Leblond, J.; Gao, H.; Petitjean, A.; Leroux, J.-C. pH-Responsive Molecular Tweezers. J. Am. Chem. Soc. 2010, 132, 8544–8545. [Google Scholar] [CrossRef]
- Viricel, W.; Mbarek, A.; Leblond, J. Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery. Angew. Chem. Int. Ed. 2015, 54, 12743–12747. [Google Scholar] [CrossRef] [PubMed]
- Viricel, W.; Poirier, S.; Mbarek, A.; Derbali, R.M.; Mayer, G.; Leblond, J. Cationic switchable lipids: pH-triggered molecular switch for siRNA delivery. Nanoscale 2017, 9, 31–36. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Controll. Release 2019, 298, 177–185. [Google Scholar] [CrossRef]
- Lou, J.; Carr, A.J.; Watson, A.J.; Mattern-Schain, S.I.; Best, M.D. Calcium-Responsive Liposomes via a Synthetic Lipid Switch. Chem. Eur. J. 2018, 24, 3599–3607. [Google Scholar] [CrossRef]
- Veremeeva, P.N.; Lapteva, V.L.; Palyulin, V.A.; Sybachin, A.V.; Yaroslavov, A.A.; Zefirov, N.S. Bispidinone-based molecular switches for construction of stimulus-sensitive liposomal containers. Tetrahedron 2014, 70, 1408–1411. [Google Scholar] [CrossRef]
- Takeuchi, J.; Ohkubo, A.; Yuasa, H. A Ring-Flippable Sugar as a Stimuli-Responsive Component of Liposomes. Chem. Asian J. 2015, 10, 586–594. [Google Scholar] [CrossRef]
- Rice, D.R.; Clear, K.J.; Smith, B.D. Imaging and therapeutic applications of zinc(ii)-dipicolylamine molecular probes for anionic biomembranes. Chem. Commun. 2016, 52, 8787–8801. [Google Scholar] [CrossRef]
- Plaunt, A.J.; Courbanou, M.B.; Cuison, K.D.; Harmatys, K.M.; Smith, B.D. Selective non-covalent triggered release from liposomes. Chem. Commun. 2012, 48, 8123–8125. [Google Scholar] [CrossRef]
- Plaunt, A.J.; Harmatys, K.M.; Hendrie, K.A.; Musso, A.J.; Smith, B.D. Chemically triggered release of 5-aminolevulinic acid from liposomes. RSC Adv. 2014, 4, 57983–57990. [Google Scholar] [CrossRef]
- Harmatys, K.M.; Musso, A.J.; Clear, K.J.; Smith, B.D. Small molecule additive enhances cell uptake of 5-aminolevulinic acid and conversion to protoporphyrin IX. Photochem. Photobiol. Sci. 2016, 15, 1408–1416. [Google Scholar] [CrossRef]
- Van Noorden, R.; Castelvecchi, D. World’s tiniest machines win chemistry Nobel. Nature 2016, 538, 152–153. [Google Scholar] [CrossRef]
- Kassem, S.; van Leeuwen, T.; Lubbe, A.S.; Wilson, M.R.; Feringa, B.L.; Leigh, D.A. Artificial molecular motors. Chem. Soc. Rev. 2017, 46, 2592–2621. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Kahlert, B. Molecular Tweezers and Clips as Synthetic Receptors. Molecular Recognition and Dynamics in Receptor−Substrate Complexes. Acc. Chem. Res. 2003, 36, 919–932. [Google Scholar] [CrossRef]
- Desrosiers, A.; Vallee-Belisle, A. Nature-inspired DNA switches: Applications in medicine. Nanomedicine 2017, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Lister, F.G.A.; Le Bailly, B.A.F.; Webb, S.J.; Clayden, J. Ligand-modulated conformational switching in a fully synthetic membrane-bound receptor. Nat. Chem. 2017, 9, 420–425. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Z.; Wang, F. Donor-acceptor-type supramolecular polymers on the basis of preorganized molecular tweezers/guest complexation. Chem. Soc. Rev. 2018, 47, 5165–5176. [Google Scholar] [CrossRef] [PubMed]








| Molecular Tweezers | Anticancer Activity | Cancer Model | Ref. |
|---|---|---|---|
 | Bisintercalator in the hexameric d(ATGCAT)2 sequence | Human colon cancer HT29 | [48] |
 | DNA fragmentation | Colon adenocarcinoma Caco-2 and HT29 | [49] |
| Chromatin condensation Caspase activation | |||
 | Interference with T and U incorporation | Leukemia | [49] |
| DNA breaks | Solid tumor models | ||
 | DNA unwinding | ND | [50] |
| Protein | Associated disease | Refs. |
|---|---|---|
| Amyloid β-protein | Alzheimer’s disease | [55,57] |
| Tau | Alzheimer’s disease, Parkinson disease | [55,57] |
| α-synuclein | Parkinson Disease, synucleinopathies | [59,60] |
| Islet amyloid polypeptide (IAPP) | Type 2 diabetes | [55,63] |
| Transthyretin (TTR) | Familial Amyloid Polyneuropathy | [55,62] |
| 14-3-3 adapter protein | Cancer, bacterial infections | [1,69] |
| Insulin | Injection-related nodular amyloidosis | [55] |
| β2-macroglobulin | Dialysis-related amyloidosis | [55] |
| Calcitonin (CT) | Medullary Carcinoma of the Thyroid | [55] |
| Polyglutamine core of HTT exon 1 | Huntington Disease | [67,68] |
| p53 | Cancer | [64] |
| Superoxide dismutase | Amyotrophic lateral sclerosis | [66] |
| Amyloid fibrils in semen | HIV | [70] |
| CryABR120G | Cardiac proteinopathies | [65] |
| Viral envelope | Ebola, Zika | [70,71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbarek, A.; Moussa, G.; Leblond Chain, J. Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips. Molecules 2019, 24, 1803. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091803
Mbarek A, Moussa G, Leblond Chain J. Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips. Molecules. 2019; 24(9):1803. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091803
Chicago/Turabian StyleMbarek, Amira, Ghina Moussa, and Jeanne Leblond Chain. 2019. "Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips" Molecules 24, no. 9: 1803. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24091803