Recent Advances in Degradable Hybrids of Biomolecules and NGs for Targeted Delivery
Abstract
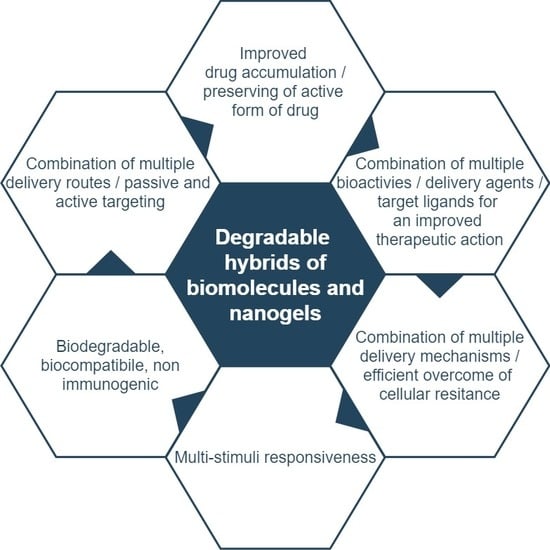
:1. Introduction
- increase in efficiency of drug loading,
- improvement in controlled release and targeted delivery,
- extension of time of nanogel systemic circulation,
- ability to absorb large amounts of various liquids,
- control of flexibility and mechanical properties of NGs, including the tunability of their sizes,
- ability to simultaneously encapsulate several drugs,
- ability of the hybrids to be loaded with various types of drugs,
- improvement in nanogel stability in aqueous solutions and in the presence of serum,
- increase in lifetime of drug presence inside the nanogel,
- introduction of multi-stimuli responsiveness to the NGs,
- ability to degrade for effective removal with kidneys or liver,
- improvement in nanogel non-toxicity and biocompatibility,
- ability to respond in a controlled, switchable way to environmental changes (on–off systems),
- possibility of remote control for effective release and delivery.
2. Design Criteria for Degradable Nanogel and Biomolecule Hybrids
2.1. Physical and Biological Properties of Biomolecule/Nanogel Hybrids
2.1.1. Morphology
- by covalent bonding to the polymer-chains network during the polymerization process,
- by covalent bioconjugation after the polymerization process, that stands with grafting the biomolecules with an application of labile bonds onto the outer surface (mainly) of the NGs,
- by physical crosslinking/entrapment/self-assembly in nanogel nets during and after the polymerization process, inside and on the outer nanogel surface.
- the design of specifically spatially organized structures of hybrid NGs, e.g., core-shells, for control of the water adsorption,
- the control of the hydrophilicity by changing the crosslinking density, e.g., by using of biomolecules as internal nanogel crosslinkers.
2.1.2. Swelling/Shrinking—Volume Phase Transition
2.1.3. Viscoelasticity
2.1.4. Biocompatibility, Degradability, and Reversibility of Volume Phase Transition
Degradation of NGs at Acidic pH
NGs Degradable under the Influence of Enzymes
NGs Degradable under the Influence of a Redox Substance
NGs Degradable under the Influence of Photochemical Reaction
2.2. Drug Loading Capacity and Controlled Release Aspects
2.3. Targeted Delivery
3. Protein and Oligonucleotide-Based Nanogel Hybrids
3.1. Polymer-Based Background
3.2. Aptamer, Oligonucleotidem, and Nanogel Cooperation
3.3. Amino Acids and Proteins in NGs
4. Mechanisms of Controlled Drug Release from Hybrid NGs
- (a)
- diffusional transport,
- (b)
- the swelling-shrinking process and degradation, and
- (c)
- presence of chemicals.
4.1. Improved Nanogel Permeability and Drug Diffusivity Relevant to Controlled Release
4.2. Improved Breaking of Thermo- or Photo-Labile Bonds
4.3. Influence of Phase Transition and Reversible Change of Conformation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sahu, P.; Kashaw, S.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorg. Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef]
- Kandil, R.; Merkel, O. Recent progress of polymeric nanogels for gene delivery. Curr. Op. Col. Interf. Sci. 2019, 39, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kapse-Mistry, S.; Govender, T.; Srivastava, R.; Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Front. Pharmacol. 2014, 5, 159–168. [Google Scholar] [PubMed]
- Dong, X.; Mumper, R. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef]
- Molina, M.; Wedepohl, S.; Miceli, E.; Calderón, M. Overcoming drug resistance with on-demand charged thermoresponsive dendritic nanogels. Nanomedicine 2017, 12, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S.; Nyström, A. Nanomedicine: Will it offer possibilities to overcome multiple drug resistance in cancer? J. Nanobiotechnol. 2016, 14, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Mbuya, V.; Gupta, N.; Tash, T. Application of nanogels in reduction of drug resistance in cancer chemotherapy. J. Chem. Pharm. Res. 2016, 8, 556–561. [Google Scholar]
- Chen, Y.; Tezcan, O.; Li, D.; Beztsinna, N.; Lou, B.; Etrych, T.; Ulbrich, K.; Metselaar, J.; Lammers, T.; Hennink, W.E. Overcoming multidrug resistance using folate receptor-targeted and pH-responsive polymeric nanogels containing covalently entrapped doxorubicin. Nanoscale 2017, 9, 10404–10419. [Google Scholar] [CrossRef]
- Patil, Y.; Toti, U.; Khdair, A.; Ma, L.; Panyam, J. Single step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials 2009, 30, 859–866. [Google Scholar] [CrossRef]
- Pramanik, D.; Campbell, N.; Das, S.; Gupta, S.; Chenna, V.; Bisht, S. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget 2012, 3, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Wan, C.; Zhang, Z.; Ruan, H.; Sun, L.; Yang, C.; Li, Y.; Qin, W.; Wang, C. Synergistic cisplatin/doxorubicin combination chemotherapy for multidrug-resistant cancer via polymeric nanogels targeting deliver. ACS Appl. Mater. Interfaces 2017, 9, 9426–9436. [Google Scholar]
- Sharma, G.; Parchur, A.; Jagtap, J.; Hansen, C.; Joshi, A. Hybrid nanostructures in targeted drug delivery. Micro. Nano. Technolog. 2019, 8, 139–158. [Google Scholar]
- Chen, X.; Zhang, X.; Guo, Y.; Zhu, Y.; Liu, X.; Chen, Z.; Wu, F. Smart supramolecular “trojan horse”-inspired nanogels for realizing light-triggered nuclear drug influx in drug-resistant cancer cells. Adv. Funct. Mater. 2019, 29, 1807772–1807781. [Google Scholar] [CrossRef]
- Manickam, P.; Pierre, M.; Jayant, R.; Nair, M.; Bhansali, S. Future of nanogels for sensing applications. In Nanogels for Biomedical Applications; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Battista, E.; Causa, F.; Netti, P. Bioengineering microgels and hydrogel microparticles for sensing biomolecular targets. Gels 2017, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Marcisz, K.; Mackiewicz, M.; Romanski, J.; Stojek, Z.; Karbarz, M. Significant, reversible change in microgel size using electrochemically induced volume phase transition. Appl. Mat. 2018, 31, 182–189. [Google Scholar] [CrossRef]
- Callejas-Fernández, J.; Estelrich, J.; Quesada-Pérez, M.; Forcada, J. RSC Nanoscience and Nanotechnology, Soft Nanoparticles for Biomedical Applications; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 133–157. [Google Scholar]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. NGs: An overview of properties, biomedical applications, and obstacles to clinical translation. J. Contr. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Vicario-de-la-Torre, M.; Forcada, J. The potential of stimuli-responsive NGs in drug and active molecule delivery for targeted therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Elena Nita, L.; Chiriac, A.P. Basic concepts and recent advances in NGs as carriers for medical applications. Drug Deliv. 2017, 25, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Raciel, D.; Rodrigues, J.; Shi, X.; Tomass, H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.; Akbari, M.; Bertassoni, L.C.; Cha, C.; Camci-Unal, G.; Dokmeci, M.; Peppas, N.; Khademhosseini, A. Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.V.P.; Torchilin, V. Intracellular delivery of proteins and peptides. Drug Deliv. 2016, 576–622. [Google Scholar]
- Vaisle, C. Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [PubMed]
- Acharya, S.; Sahoo, S. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.; Kevin Ming, K.; Chan, W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Mat. 2009, 4, 707–715. [Google Scholar] [CrossRef]
- Tanaka, T. Kinetics of phase transition in polymer gels. Physica A 1986, 140, 261–268. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A.; Loftsson, T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2012, 428, 152–163. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug. Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro-and nanogels with labile crosslinks–from synthesis to biomedical applications. Chem Soc Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desale, S.S.; Cohen, S.M.; Zhao, Y.; Kabanov, A.V.; Bronich, T.K. Biodegradable hybrid polymer micelles for combination drug therapy in ovarian cancer. J. Control. Release. 2013, 171, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.L.; Herlihy, K.P.; Napier, M.E.; DeSimone, J.M. PRINT: A novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 2011, 44, 990–999. [Google Scholar] [CrossRef]
- Wu, H.Q.; Wang, C.C. Biodegradable smart NGs: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef]
- Eslami, P.; Rossi, F.; Fedeli, S. Hybrid nanogels: stealth and biocompatible structures for drug delivery applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef]
- Lyon, L.A.; Serpe, M.J. Hydrogel Micro and Nanoparticles; Wiley-VCH: Weincheim, Germany, 2012; pp. 2013–2226. [Google Scholar]
- Gao, Y.; Xie, J.; Chen, H. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol. Adv. 2014, 32, 761–777. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [Green Version]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.J.; Hoffman, J.M.; Uto, K.; Aoyag, T. Smart Biomaterials; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef]
- Wang, F.; Gao, W.; Thamphiwatana, S.; Luk, B.T.; Angsantikul, P.; Zhang, Q.; Hu, C.M.J.; Fang, R.H.; Copp, J.A.; Pornpattananangkul, D.; et al. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv. Mater. 2015, 27, 3437–3443. [Google Scholar] [CrossRef]
- Zhao, J.; Su, H.; Vansuch, G.E.; Liu, Z.; Salaita, K.; Dyer, R.B. Localized nanoscale heating leads to ultrafast hydrogel volume-phase transition. ACS Nano. 2019, 13, 515–525. [Google Scholar] [CrossRef]
- Liwinska, W.; Symonowicz, M.; Stanislawska, I.; Łyp, M.; Stojek, Z.; Zabost, E. Environmentally sensitive nanohydrogels decorated with a three-strand oligonucleotide helix for controlled loading and prolonged release of intercalators. RSC Adv. 2016, 69, 1045–1059. [Google Scholar] [CrossRef]
- Liwinska, W.; Stanislawska, I.; Lyp, M.; Stojek, Z.; Zabost, E. Switchable conformational changes of DNA nanogel shells containing disulfide–DNA hybrids for controlled drug release and efficient anticancer action. RSC Advances 2019, 9, 13736–13748. [Google Scholar] [CrossRef]
- Liwinska, W.; Stanislawska, I.; Lyp, M.; Mackiewicz, M.; Stojek, Z.; Zabost, E. Degradable nanogel drug carrier crosslinked with three-oligonucleotide hybrids for two-way drug release in mild- and high hyperthermia treatment. J. Mater. Chem. B 2017, 5, 4713–4724. [Google Scholar] [CrossRef]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Wu, T.; Zrimsek, A.B.; Bykov, S.V.; Jakubek, R.S.; Asher, A.S. Hydrophobic collapse initiates the poly(N-isopropylacrylamide) volume phase transition reaction coordinate. J. Phys. Chem. B 2018, 122, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Naficy, S.; Brown, H.R.; Razal, J. Progress toward robust polymer hydrogels. Aust. J. Chem. 2011, 64, 1007–1025. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart Materials for Drug Delivery Vol.1; RCS Publishing: Cambridge, UK, 2013. [Google Scholar]
- Conde, J. Handbook of Nanomaterials for Cancer Theranostics; Elsevier Inc.: Oxford, MS, USA, 2018. [Google Scholar]
- Patel, V.R.; Amiji, M.M. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res. 1996, 13, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Esenaliev, R. PLGA Nanoparticles for ultrasound-mediated gene delivery to solid tumors. J. Drug. Deliv. 2012, 2012, 1–20. [Google Scholar] [CrossRef]
- Matsumoto, N.M.; González-Toro, D.C.; Chacko, R.T.; Maynard, H.D.; Thayumanavan, S. Synthesis of nanogel–protein conjugates. Polym. Chem. 2013, 4, 2464–2469. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, M.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J. In situ forming reduction-sensitive degradable NGs for facile loading and triggered intracellular release of proteins. Biomacromol. 2013, 14, 1214–1222. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Xiong, W.; Pei, Y.; Li, B.; Chen, Y. NGs fabricated from bovine serum albumin and chitosan via self-assembly for delivery of anticancer drug. Coll. Surf. B Biointerf. 2016, 1, 107–113. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Oberoi, H.S.; Cohen, S.M.; Kabanov, A.V.; Bronich, T.K. Folate-decorated NGs for targeted therapy of ovarian cancer. Biomater. 2011, 32, 5417–5426. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Ueda, S.; Nishikawa, H.; Kitano, S.; Hirayama, M.; Ikeda, H.; Toyoda, H.; Tanaka, K.; Kanai, M.; Takabayashi, A.; et al. Antibody responses against NY-ESO-1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine 2009, 16, 6854–6861. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.; Lambrecht, B.N.; et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Park, K.H. Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels. Carbohydr Polym. 2016, 36, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv. Mat. 2017, 29, 1701170–1701178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-stabilized hyaluronate nanogel for intracellular codelivery of doxorubicin and cisplatin to osteosarcoma. Adv. Sci. 2018, 5, 1700821–1700833. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.; Li, D.; Lao, Y.; Liu, D.; Li, M.; Chen, X. Tumor microenvironment-responsive hyaluronate-calcium carbonate hybrid nanoparticle enables effective chemotherapy for primary and advanced osteosarcoma. Nano. Res. 2018, 11, 4806–4822. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, F.; Xu, W.; Chen, J.; Hou, J.; Wang, C.; Ding, J.; Chen, X. Mucoadhesive cationic polypeptide nanogel with enhanced penetration for efficient intravesical chemotherapy of bladder cancer. Adv. Sci. 2018, 5, 1800004–1800014. [Google Scholar] [CrossRef]
- Guo, H.; Xu, W.; Chen, J.; Yan, L.; Ding, J.; Hou, Y.; Chen, X. Positively charged polypeptide nanogel enhances mucoadhesion and penetrability of 10-hydroxycamptothecin in orthotopic bladder carcinoma. J. Control. Release 2017, 259, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Yang, M.; Feng, X.; Wang, Y.; Chang, F.; Ding, J. Reduction-responsive polypeptide nanogel for intracellular drug delivery in relieving collagen-induced arthritis, ACS Biomater. Sci. Eng. 2018, 4, 4154–4162. [Google Scholar]
- Shi, B.; Huang, K.; Ding, J.; Xu, W.; Yang, Y.; Liu, H.; Yan, L.; Chen, X. Intracellularly swollen polypeptide nanogel assists hepatoma chemotherapy. Theranostics 2017, 7, 703–716. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Xu, W.; Sun, T.; Xiao, H.; Zhuang, X.; Chen, X. Receptor and microenvironment dual-recognizable nanogel for targeted chemotherapy of highly metastatic malignancy. Nano. Lett. 2017, 17, 4526–4533. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Kaniewska, K.; Romanski, J.; Augustin, E.; Stojek, Z.; Karbarz, M. Stable and degradable microgels linked with cystine for storing and environmentally triggered release of drugs. J. Mater. Chem. B 2015, 3, 7262–7270. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, W.; Zhou, S. Engineering oligo(ethylene glycol)-based thermosensitive microgels for drug delivery applications. Polymer 2010, 51, 3926–3933. [Google Scholar] [CrossRef]
- Li, D.; van Nostrum, C.F.; Mastrobattis, E.; Vermonden, T.; Hennink, W.E. NGs for intracellular delivery of biotherapeutics. J. Control. Release 2017, 259, 16–28. [Google Scholar] [CrossRef]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mat. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Li, Y. Softer zwitterionic NGs for longer circulation and lower splenic accumulation. ACS Nano 2012, 6, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Maheshwari, R.; Kiick, K.L. Polymer-based therapeutic. Macromolecules 2009, 42, 3–13. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Du, J.Z.; Song, W.J.; Wang, F.; Yang, X.Z.; Xiong, M.H.; Wang, J. Biocompatible and functionalizable polyphosphate nanogel with a branched structure. J. Mater. Chem. 2012, 22, 9322–9329. [Google Scholar] [CrossRef]
- Coll Ferrer, M.C.; Sobolewski, P.; Composto, R.J.; Eckmann, D.M. Cellular uptake and intracellular cargo release from dextran based nanogel drug carriers. J. Nanotechnol. Eng. Med. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Urakami, H.; Hentschel, J.; Seetho, K.; Zeng, H.; Chawla, K.; Guan, Z. Surfactant-free synthesis of biodegradable, biocompatible, and stimuli-responsive cationic nanogel particles. Biomacromolecules 2013, 14, 3682–3688. [Google Scholar] [CrossRef]
- Kuckling, D. Stimuli Responsive Gels; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Oishi, M.; Nagasaki, Y. Stimuli-responsive smart nanogels for cancer diagnostics and therapy. Nanomedicine 2010, 5, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.; Ho, V.T.; Huang, W.; Huang, Y.; Chern, C.; Chiu, H. Dual stimuli-responsive polymeric hollow NGs designed as carriers for intracellular triggered drug release. Langmuir 2012, 28, 15056–15064. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Heo, G.S.; Lim, S.; Sun, G.; Wooley, K.L. Polymeric nanostructures for imaging and therapy. Chem Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef]
- Bulmus, V.; Chan, Y. Synthesis and characterization of degradable p(HEMA) microgels: Use of acid-labile crosslinkers. Macromol. Biosci. 2007, 7, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Witting, M.; Haag, R. Surfactant free preparation of biodegradable dendritic polyglycerol nanogels by inverse nanoprecipitation for encapsulation and release of pharmaceutical biomacromolecules. J. Control. Release 2013, 169, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Khondee, S.; Berkland, C. Poly(N-vinylformamide) nanogels capable of pH-sensitive protein release. Macromolecules 2008, 41, 6546–6554. [Google Scholar] [CrossRef]
- Sunasee, R.; Wattanaarsakit, P.; Narain, R. Biodegradable and non-toxic cationic nanogels as non-viral gene delivery systems. Bioconjugate Chem. 2012, 23, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Washburn, N.R.; Matyjaszewski, K. Synthesis by AGET ATRP of degradable nanogel precursors for n situ formation of nanostructured Hyaluronic Acid Hydrogel. Biomacromolecules 2009, 10, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.B.; Naeye, K.; Smedt, D. Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery. Adv. Funct. Mater. 2009, 19, 1406–1415. [Google Scholar] [CrossRef]
- Patenaude, M.; Hoare, T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules 2012, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, D.; Kootala, S.; Hilborn, J. Orthogonal chemoselective assembly of hyaluronic acid networks and nanogels for drug delivery. Macromolecules 2013, 46, 4105–4113. [Google Scholar] [CrossRef]
- Wang, L.; Cao, W.; Yi, Y. Dual redox responsive co-assemblies of diselenide-containing block copolymers and polymer lipids. Langmuir 2014, 30, 5628–5635. [Google Scholar] [PubMed]
- Wang, Y.; Wu, J.; Wang, L. Engineering nanoscopic hydrogels via photo-crosslinking salt induced polymer assembly for targeted drug delivery. Chem. Commun. 2010, 46, 3520–3522. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Jiang, X. Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. J. Control. Release 2015, 205, 206–217. [Google Scholar] [CrossRef]
- Stanisławska, I.; Witek, B.; Łyp, M.; Rochon-Szmejchel, D.; Wróbel, A.; Fronczyk, W.; Kamińska, A.; Kołątaj, A.; Załuski, D. Effects of glutathione on hydrolytic enzyme activity in the Mouse hepatocytes. Adv. Exp. Med. Biol. Clin. Experim. Biomed. 2018, 1116, 81–87. [Google Scholar]
- Witek, B.; Rochon-Szmejchel, D.; Stanisławska, I.; Łyp, M.; Wróbel, K.; Zapała, A.; Kamińska, A.; Kołątaj, A. Activities of lysosomal enzymes in alloxan-induced diabetes in the Mouse. Exp. Med. Biol. Neuros. Resp. 2018, 1040, 73–81. [Google Scholar]
- Oh, J.K.; Siegwart, D.J.; Matyjaszewski, K. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: Synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Chacko, R.T.; Jiwpanich, S.; Bickerton, S.; Babu, R.P.; Thayumanavan, S. Self-cross-linked polymer nanogels: A versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010, 132, 17227–17235. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, Y.; Ren, H.; Xu, H.; Lib, Z.; Zhang, X. Selenium-containing block copolymers and their oxidation-responsive aggregates. Polym. Chem. 2010, 1, 1609–1614. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Dual stimuli-responsive poly(2-hydroxyethyl methacrylate-co-methacrylic acid) microgels based on photo-cleavable cross-linkers: pH-dependent swelling and light-induced degradation. Macromolecules 2011, 44, 9758–9772. [Google Scholar] [CrossRef]
- He, J.B.; Yan, L.; Zhao, Y. Both core- and shell-cross-linked nanogels: photoinduced size change, intraparticle LCST, and interparticle UCST thermal behaviors. Langmuir 2010, 27, 436–444. [Google Scholar] [CrossRef]
- Gu, Z. Bioinspired and Biomimetic Polymer Systems for Drug and Gene Delivery; Wiley-VCH: Weincheim, Germany, 2015. [Google Scholar]
- Yan, M.; Ge, J.; Liu, Z.; Ouyang, P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J. Am. Chem. Soc. 2006, 128, 11008–11009. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.H.; Kim, S.H.; Park, T.G. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Swelling induced physical disruption of endosome by cold shock. J. Control. Release 2008, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Halabi, N.; Alsalloum, G. Nanogels as novel drug delivery systems—A Review. J. Pharm. Pharm. Res. 2017, 1, 1–5. [Google Scholar]
- Kong, H.Y.; Byun, J. Screening and characterization of a novel RNA aptamer that specifically binds to human prostatic acid phosphatase and human prostate cancer cells. Biomol. Ther. 2013, 21, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wooram, P.; Kyoung, K. Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Eur. J. Pharm. Sci. 2010, 40, 367–375. [Google Scholar]
- Zhu, G.; Cansiz, S.; Tan, W. Nuclease-resistant synthetic drug-DNA adducts: Programmable drug-DNA conjugation for targeted anticancer drug delivery. NPG Asia Mat. 2015, 7, e169. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Aravind, A.; Jeyamohan, P.; Nair, Z. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Li, N.; Yao, Y.; Guo, C.; Ge, Y.; Wang, J. Polypeptide nanogels with different functional cores promote chemotherapy of lung carcinoma. Front. Pharmacol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Kersey, F.R.; Merkel, T.J.; Perry, J.L. Effect of aspect ratio and deformability on nanoparticle extravasation through nanopores. Langmuir 2012, 28, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Karbarz, M.; Mackiewicz, M.; Kaniewska, K.; Marcisz, K.; Stojek, Z. Recent developments in design and functionalization of micro- and nanostructural environmentally-sensitive hydrogels based on N-isopropylacrylamide. Appl. Mat. Today 2017, 9, 516–532. [Google Scholar] [CrossRef]
- Bian, S.; Zheng, J.; Tang, X.; Yi, D.; Wang, Y.; Yang, W. One-pot synthesis of redox-labile polymer capsules via emulsion droplet-mediated precipitation polymerization. Chem. Mater. 2015, 25, 1262–1268. [Google Scholar] [CrossRef]
- Mallikaratchy, P. Evolution of complex target SELEX to identify aptamers against mammalian cell-surface antigen. Molecules 2017, 22, 215. [Google Scholar] [CrossRef]
- Cansiz, S.; Zhang, L.; Wu, C.; Wu, Y.; Teng, I.; Hou, W.; Wang, Y.; Wan, S.; Cai, R.; Jin, C.; et al. DNA Aptamer Based Nanodrugs: Molecular Engineering for Efficiency. Chem. Asian. J. 2015, 10, 2084–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Wang, H.; Chen, Y.; Zhang, X.; Zhu, H.; Yang, C.; Yang, R. Molecular aptamers for drug delivery. Trends Biotechnol. 2011, 29, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Vu, C.; Rotkrua, P.; Soontornworajit, B.; Tantirungrotechai, Y. Effect of PDGF-B aptamer on PDGFRβ/PDGF-B interaction: Molecular dynamics study. J. Mol. Graph. Model 2018, 82, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Latchoumanin, O.; Bagdesar, M.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamer-based therapeutic approaches to target cancer stem cells. Theranostics 2017, 7, 3948–3961. [Google Scholar] [CrossRef]
- Baek, S.; Lee, K.; Park, Y.; Oh, D.K.; Oh, S.; Kim, K.; Kim, D. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control Release 2014, 28, 234–242. [Google Scholar] [CrossRef]
- Xiao, Z.; Farokhzad, O.C. Aptamer-functionalized nanoparticles for medical applications: challenges and opportunities. ACS Nano. 2012, 22, 3670–3676. [Google Scholar] [CrossRef]
- Sivakumar, B.; Aswathy, R.G.; Nagaoka, Y.; Iwai, S.; Venugopal, K.; Kato, K.; Yoshida, Y.; Maekawa, T.; Kumar, D. Aptamer conjugated theragnostic multifunctional magnetic nanoparticles as a nanoplatform for pancreatic cancer therapy. RSC Adv. 2013, 3, 20579–20598. [Google Scholar] [CrossRef]
- Lale, S.V.; Aravind, A.; Kumar, D.S.; Koul, V. AS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromol. 2014, 151737–151752. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.T.; Fowler, A.J.; Garmon, C.B.; Fessler, A.B.; Ogle, J.D.; Grover, K.R.; Allen, B.C.; Williams, C.D.; Zhou, R.; Yazdanifar, M.; et al. Treatment of pancreatic ductal adenocarcinoma with tumor antigen specific-targeted delivery of paclitaxel loaded PLGA nanoparticles. BMC Cancer. 2018, 23, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Sayari, E.; Dinarvand, M.; Amini, M.; Azhdarzadeh, M.; Mollarazi, E.; Ghasemi, Z.; Atyabi, F. MUC1 aptamer conjugated to chitosan nanoparticles, an efficient targeted carrier designed for anticancer SN38 delivery. Int. J. Pharm. 2014, 473, 304–315. [Google Scholar] [CrossRef]
- Alibolandi, M.; Ramezani, M.; Sadeghi, F.; Abnous, K.; Hadizadeh, F. Epithelial cell adhesion molecule aptamer conjugated PEG-PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro. Int. J. Pharm. 2015, 479, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiang, D.; Shigdar, S.; Yang, W.; Li, Q.; Lin, J.; Liu, K.; Duan, W. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int. J. Nanomedicine 2014, 9, 1083–1096. [Google Scholar] [PubMed] [Green Version]
- Bagalkot, V.; Farokhzad, O.C.; Langer, R. An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew. Chem. 2006, 45, 8149–8152. [Google Scholar] [CrossRef]
- Xing, H.; Tang, L.; Yan, X. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef]
- Huang, F.; Shangguan, T.W. Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. Chem. Bio. Chem. 2009, 10, 862–868. [Google Scholar] [CrossRef]
- Wang, J.; Sefah, K.; Tan, H. Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem. Asian, J. 2014, 8, 2417–2422. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.F.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA. 2011, 105, 2586–2591. [Google Scholar] [CrossRef]
- Huang, F.; You, M.; Chen, Z.; Tan, W. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem. Commun. 2014, 50, 3103–3105. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Ramezani, M.; Hadizadeh, F. AS1411 aptamer-decorated biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non-small cell lung cancer in vitro. J. Pharm Sci. 2016, 105, 1741–1750. [Google Scholar] [CrossRef]
- Thorek, D.L.; Elias, D.R.; Tsourkas, A. Comparative analysis of nanoparticle-antibody conjugations: Carbodiimide versus click chemistry. Mol Imaging. 2009, 8, 221–229. [Google Scholar] [CrossRef]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Hasegawa, U.; Sawada, S.; Shimizu, T.; Kishida, T.; Otsuji, E.; Mazda, O. Raspberry-like assembly of cross-linked nanogels for protein delivery. J. Control. Release 2009, 140, 312–317. [Google Scholar] [CrossRef]
- Nagahama, K.; Ouchi, T.; Ohya, Y. Biodegradable nanogels prepared by self-assembly of poly (l-lactide)-grafted dextran: Entrapment and release of proteins. Macromol Biosci. 2008, 8, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Van Thienen, T.; Raemdonck, K.; Demeester, J.; De Smedt, S. Protein release from biodegradable dextran NGs. Langmuir 2007, 23, 9794–9801. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Srinivasan, A.; Bencherif, S.A.; Karunanidhi, A.; Oh, J.K.; Vaidya, S.; Jin, R.; Hollinger, J.O.; Matyjaszewski, K. Cellular uptake of functional nanogels prepared by inverse miniemulsion ATRP with encapsulated proteins, carbohydrates, and gold nanoparticles. Biomacromolecules 2009, 10, 2300–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wutzel, H.; Richter, F.H.; Li, Y.; Sheiko, S.S.; Klok, H. Poly [N-(2-hydroxypropyl) methacrylamide] nanogels by RAFT polymerization in inverse emulsion. Polym. Chem. 2014, 5, 1711–1719. [Google Scholar] [CrossRef]
- Ouchida, N.; Kiyono, Y. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert. Rev. Vaccines 2017, 16, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomedicine 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer Verlag GMBH: Heidelberg, Germany, 2014; pp. 19–43. [Google Scholar]
- Salim, M.; Minamikawa, H.; Sugimur, A.; Hashim, R. Amphiphilic designer nanocarriers for controlled release: from drug delivery to diagnostics. Med. Chem Comm. 2014, 11, 1602–1618. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef] [PubMed]








| Type of Hybrid Nanogel | Type of Response-Stimulus | Type of Released Drug/Compound | Reference |
|---|---|---|---|
| Chitosan/PEO | pH | Amoxicillin, metronidazole | [58] |
| Y-gel-aptamer/DNA | redox-GSH | Aptamer | [21] |
| MUC1 aptamer/PLGA | MUC1 protein, ultrasounds, | Aptamer | [59] |
| FA/pullulan | Hyaluronic receptors | Doxorubicin, DOX | [37] |
| Protein/PEGMA-co-PDSMA | redox-GSH | BSA protein | [60] |
| Protein/PEG-P(HEMA-co-AC) | redox-DTT | Cytochrome C | [61] |
| BSA protein/chitosan | pH | DOX | [62] |
| FA/PEO-b-PMA | Folate receptor, pH | CDDP, DOX | [63] |
| Antibodies vaccine/cholesteryl pullulan | NY-ESO-1 and HER2 antigens | Antibodies | [64] |
| imidazoquinoline-based TLR7/8/poly(mTEGMA-b-HEMAm) | pH | TLR7/8 agonist | [65] |
| antibody/PEI/DNA/HA | CD44 receptor, surface charge | Aptamer | [66] |
| SN NPs with -SS-crosslinkers | redox-GSH, pH | DOX | [67] |
| CDDP crosslinked-HA | pH | DOX, CDDP | [68] |
| CaCO3-crosslinked HA | pH | DOX | [69] |
| sarcoma-targeting peptide-SS-crosslinked polypeptide | Sarcoma receptors, redox-GSH | SHK | [70] |
| SS-crosslinked (PLL–P(LP-co-LC)) | redox-GSH | HCPT | [71,72] |
| SS-crosslinked (mPEG–P(LP-co-LC)) | redox-GSH | MTX | [73] |
| Polypeptide-based | redox-GSH, pH | DOX | [74] |
| nanogels with PBA and MP | sialyl epitopes receptors, pH | DOX | [75] |
| Tri-segment oligonucleotide/PNIPA-AAc | pH, temperature, surface charge | DOX | [50] |
| Oligonucleotide-crosslinked/PNIPA-AAc | pH, temperature, surface charge | DOX | [51] |
| Oligonucleotide-SS-/PNIPA-AAc | redox-GSH, temperature, pH surface charge | DOX | [52] |
| Linker Type | Chemical Structure | Degradation Conditions | Ref |
|---|---|---|---|
| Acetalic linker |  | Hydrolysis in acidic medium, pH = 5 | [60] |
| Ketal linker |  | Hydrolysis in acidic medium, pH = 5.5 | [61] |
| Ester linker |  | Hydrolysis below physiological pH | [62] |
| Vinyl ether linker |  | Hydrolysis in acidic medium, pH <5 | [63] |
| Linker based on ortho-nitrobenzyl ester |  | Hydrolysis under the influence of UV 315–390 nm | [64] |
| Linker based on disulfide or diselenide bridges |   | Hydrolysis in the presence of GSHcarboxyethylphosphine tris (TCEP), and Dithiothreitol (DTT) | [65,66,67] |
| Phosphoester linker |  | Hydrolysis in the presence of phosphatase or phospholipase enzyme | [68] |
| Aptamer Type | Cancer Type | Type of Drug | Type of Nanogel | Ref. |
|---|---|---|---|---|
| RNA/PSMA | Prostate cancer | Docetaxel | PLGA-b-PEG | [128] |
| RNA/PSMA | Prostate cancer | Doxorubicin | Dendrimers | [129] |
| RNA/PSMA | Prostate cancer | Docetaxel | PLGA-liposomes | [115] |
| DNA/AS1411 | Pancreatic cancer | Curcumin and gemcitabine | PLGA/magnetic | [130] |
| DNA/AS1411 | Breast, pancreatic cancer | Doxorubicin | pPEGMA-PCL-pPEGMA | [131] |
| DNA/MUC1 | Pancreatic cancer | Paclitaxel | PLGA | [132] |
| DNA/MUC1 | Colon cancer | SN-38 | Chitosan | [133] |
| RNA/EpCAM | Breast cancer | Doxorubicin | PLGA/PEG | [134] |
| RNA/EpCAM | Breast cancer | Curcumin | PLGA-lecithin-PEG | [135] |
| PDNA/PGDF-B | Ovarian cancer | PGDF-B | Streptavidin-coated polystyrene, poloxamer | [136] |
| RNA/Ep | Breast cancer | siRNA | PEI | [136] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanislawska, I.; Liwinska, W.; Lyp, M.; Stojek, Z.; Zabost, E. Recent Advances in Degradable Hybrids of Biomolecules and NGs for Targeted Delivery. Molecules 2019, 24, 1873. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24101873
Stanislawska I, Liwinska W, Lyp M, Stojek Z, Zabost E. Recent Advances in Degradable Hybrids of Biomolecules and NGs for Targeted Delivery. Molecules. 2019; 24(10):1873. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24101873
Chicago/Turabian StyleStanislawska, Iwona, Wioletta Liwinska, Marek Lyp, Zbigniew Stojek, and Ewelina Zabost. 2019. "Recent Advances in Degradable Hybrids of Biomolecules and NGs for Targeted Delivery" Molecules 24, no. 10: 1873. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24101873