Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield and Tyrosinase Inhibitory Activity of M. zapota Bark
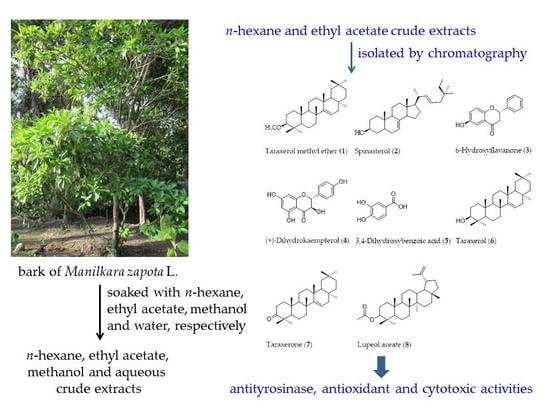
2.2. Identification of Compounds 1–8
2.3. Spectroscopic Data of Compounds 1–8
2.4. Mushroom Tyrosinase Inhibitory Activity of Compounds 1–8
2.5. Kinetic Inhibition of Compounds 1–8 on Tyrosinase Inhibitory Activity
2.6. Antioxidant Activities of Compounds 1–8
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.6.3. FRAP Activity
2.7. Cytotoxicity of Compounds 1–8
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Chemicals and Reagents
4.3. Plant Material
4.4. Extraction of M. zapota Bark
4.5. Bioactivity-Guided Isolation and Identification of M. zapota Bark
4.6. Mushroom Tyrosinase Inhibitory Assay
4.7. Kinetic Analysis of Tyrosinase Inhibitory Activity
4.8. Antioxidant Assays
4.8.1. DPPH Radical Scavenging Assay
4.8.2. ABTS Radical Scavenging Assay
4.8.3. FRAP Assay
4.9. Cytotoxicity Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Review: Oxidative stress and disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Derm. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.J.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the l-tyrosine supply for melanogenesis. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Videira, I.F.D.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. Anais Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Prota, G. An introduction to melanin research. In Melanins and Melanogenesis; Prota, G., Ed.; Academic Press: San Diego, CA, USA, 1992; pp. 1–9. [Google Scholar]
- Rojekar, M.V.; Sawant, S.D. A short review of pigmentation disorders in systemic diseases. J. Pigment. Disord. 2015, 2, 174–176. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Oberley, T.D. Oxidative damage and cancer. Am. J. Pathol. 2002, 160, 403–408. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Morte, D.D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Gelband, H.; Jha, P.; Sankaranarayanan, R.; Horton, S. Cancer, 3rd ed.; The International Bank for Reconstruction and Development, The World Bank: Washington, DC, USA, 2015; pp. 1–341. [Google Scholar]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. BioMedicine 2017, 7, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity - exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [PubMed]
- Kaur, R.; Singh, J.; Singh, G.; Kaur, H. Anticancer plants: A review. J. Nat. Prod. Plant. Resour. 2011, 1, 131–136. [Google Scholar]
- Haque, M.U.; Ferdiousi, N.; Sajon, S.R. Anti-cancer agents derived from plant and dietary sources: A review. Int. J. Pharmacogn. 2016, 3, 55–66. [Google Scholar]
- Chantaranothai, P. Sapotaceae. In Flora of Thailand; Santisuk, T., Balslev, H., Eds.; The Forest Herbarium, National Park, Wildlife and Plant Conservation Department: Bangkok, Thailand, 2014; pp. 610–655. [Google Scholar]
- Morton, J.F. Sapodilla. In Fruits of Warm Climates; Morton, J.F., Ed.; Creative Resource Systems: High Point, NC, USA, 1987; pp. 393–398. [Google Scholar]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Fayek, N.M.; Monem, A.R.A.; Mossa, M.Y.; Meselhy, M.R. New triterpenoid acyl derivatives and biological study of Manilkara zapota (L.) Van Royen fruits. Pharmacogn. Res. 2013, 5, 55–59. [Google Scholar]
- Fayek, N.M.; Monem, A.R.A.; Mossa, M.Y.; Meselhy, M.R.; Shazly, A.H. Chemical and biological study of Manilkara zapota (L.) Van Royen leaves (Sapotaceae) cultivated in Egypt. Pharmacogn. Res. 2012, 4, 85–91. [Google Scholar]
- Kaneria, M.; Chanda, S. Evaluation of antioxidant and antimicrobial properties of Manilkara zapota L. (chiku) leaves by sequential soxhlet extraction method. Asian Pac. J. Trop. Biomed. 2012, 2, S1526–S1533. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A. Acaricidal activity of aqueous extract and synthesized silver nanoparticles from Manilkara zapota against Rhipicephalus (Boophilus) microplus. Res. Vet. Sci. 2012, 93, 303–309. [Google Scholar] [CrossRef]
- Rashid, M.M.; Hossain, M.I.; Osman, M.A.; Aziz, M.A.; Habib, M.R.; Karim, M.R. Evaluation of antitumor activity of Manilkara zapota leaves against Ehrlich ascites carcinoma in mice. Environ. Exp. Biol. 2014, 12, 131–135. [Google Scholar]
- Toze, F.A.A.; Fomani, M.; Nouga, A.B.; Chouna, J.R.; Kouam, B.; Waffo, A.F.K.; Wansi, J.D. Taraxastane and lupane triterpenoids from the bark of Manilkara zapota. Int. Res. J. Pure Appl. Chem. 2015, 7, 157–164. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Bano, M.; Ahmed, B. Manilkara zapota (L.) P. Royen (Sapodilla): A review. IJARIIT 2017, 3, 1364–1371. [Google Scholar]
- Bashir, S. Pharmacological importance of Manilkara zapota and its bioactive constituents. Bol. Latinoam. Caribe Plantas 2019, 18, 347–358. [Google Scholar]
- Rao, G.V.; Sahoo, M.R.; Madhavi, M.S.L.; Mukhopadhyay, T. Phytoconstituents from the leaves and seeds of Manilkara zapota Linn. Der. Pharm. Lett. 2014, 6, 69–73. [Google Scholar]
- Obara, T.; Abe, S. Structure of sawamilletin from sawa millet (Barnyard grass) oil. Bull. Agric. Chem. Soc. Jpn. 1957, 21, 388–389. [Google Scholar] [CrossRef]
- Simelane, M.B.C.; Shonhai, A.; Shode, F.O.; Smith, P.; Singh, M.; Opoku, A.R. Anti-plasmodial activity of some Zulu medicinal plants and of some triterpenes isolated from them. Molecules 2013, 18, 12313–12323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, D.H.; Zhao, B.T.; Le, D.D.; Min, B.S.; Kim, Y.H.; Woo, M.H. Triterpenoids and sterols from the grains of Echinochloa utilis Ohwi & Yabuno and their cytotoxic activity. Biomed. Pharmacother. 2017, 93, 202–207. [Google Scholar] [PubMed]
- Choi, S.Z.; Lee, S.O.; Choi, S.U.; Lee, K.R. A new sesquiterpene hydroperoxide from the aerial parts of Aster oharai. Arch. Pharm. Res. 2003, 26, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Guria, M.; Mitra, P.; Ghosh, T.; Gupta, S.; Basu, B.; Mitra, P.K. 3,4-Dihydroxybenzoic acid isolated from the leaves of Ageratum conyzoides L. Eur. J. Biotechnol. Biosci. 2013, 1, 25–28. [Google Scholar]
- Herath, W.; Mikell, J.R.; Hale, A.L.; Ferreira, D.; Khan, I.A. Microbial metabolism part 9. Structure and antioxidant significance of the metabolites of 5,7-dihydroxyflavone (chrysin), and 5- and 6-hydroxyflavones. Chem. Pharm. Bull. 2008, 56, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.E.; Yin, X.F.; Choi, D.B.; Lim, S.S.; Kang, I.J.; Shim, J.H. Inhibitory activity of aromadendrin from prickly pear (Opuntia ficus-indica) root on aldose reductase and the formation of advanced glycation end products. Food Sci. Biotechnol. 2011, 20, 1283–1288. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.T.; Yang, J.H.; Baek, N.I.; Kim, D.K. Acetylcholinesterase inhibitors from the twigs of Vaccinium oldhami Miquel. Arch. Pharm. Res. 2004, 27, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Mawa, S.; Said, I.M. Chemical constituents of Garcinia prainiana. Sains Malays 2012, 41, 585–590. [Google Scholar]
- Prachayasittikul, S.; Saraban, P.; Cherdtrakulkiat, R.; Ruchirawat, S.; Prachayasittikul, V. New bioactive triterpenoids and antimalarial activity of Diospyros rubra Lec. Excli J. 2010, 9, 1–10. [Google Scholar] [PubMed]
- Seong, Z.K.; Kim, H.S.; Won, Y.M.; Kim, J.L.; Song, H.H.; Kim, D.Y.; Oh, S.R.; Cho, H.W.; Cho, J.H.; Lee, H.K. Phenylacylphenol derivatives with anti-melanogenic activity from Stewartia pseudocamellia. Arch. Pharm. Res. 2016, 39, 636–645. [Google Scholar] [CrossRef]
- Viswanadh, G.S.; Ramaiah, P.A.; Laatsch, H.; Maskey, R. Chemical constituents of the heartwood and bark of Homonoia riparia. J. Trop. Med. Plants 2006, 7, 267–273. [Google Scholar]
- Chen, C.Y.; Lin, L.C.; Yang, W.F.; Bordon, J.; Wang, H.M.D. An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Curr. Org. Chem. 2015, 19, 4–18. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Muῆoz-Moῆoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Dat, L.D.; Thao, N.P.; Luyen, B.T.T.; Tai, B.H.; Jeong, M.H.; Woo, M.H.; Kim, Y.H. Identification of six new lupane-type triterpenoids from Acanthopanax koreanum leaves and their tyrosinase inhibitory activities. Bioorg. Med. Chem. Lett. 2016, 26, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Tan, H.Y.; Chen, J.; Wang, M. Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure-activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Muῆoz-Muῆoz, J.L.; Garcia-Molina, F.; Berna, J.; Garcia-Ruiz, P.A.; Varon, R.; Tudela, J.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Kinetic characterization of o-aminophenols and aromatic o-diamines as suicide substrates of tyrosinase. Biochim. Biophys. Acta 2012, 1824, 647–655. [Google Scholar]
- Chen, Q.X.; Song, K.K.; Wang, Q.; Huang, H. Inhibitory effects on mushroom tyrosinase by some alkylbenzaldehydes. J. Enzym. Inhib. Med. Chem. 2003, 18, 491–496. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2011, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Dej-adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and antimicrobial activities from Thai medicinal plants. Arch. Pharm. Res. 2014, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Comp. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Nugitrangson, P.; Puthong, S.; Iempridee, T.; Pimtong, W.; Pornpakakul, S.; Chanchao, C. In vitro and in vivo characterization of the anticancer activity of Thai stingless bee (Tetragonula laeviceps) cerumen. Exp. Biol. Med. 2016, 241, 166–176. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Crude Extract | IC50 (μg/mL) |
|---|---|
| n-Hexane | 557.03 ± 24.13 c |
| EtOAc | 191.69 ± 6.05 b |
| MeOH | 844.22 ± 26.27 d |
| Aqueous | 1660.24 ± 11.29 e |
| Kojic acid * | 41.06 ± 3.38 a |
| α-Arbutin * | 57.54 ± 2.54 a |
| Compound | IC50 (μM) | |
|---|---|---|
| Monophenolase Inhibitory Activity | Diphenolase Inhibitory Activity | |
| Taraxerol methyl ether (1) | 325.55 ± 0.45 h | 339.33 ± 0.12 g |
| Spinasterol (2) | 722.44 ± 0.48 j | 973.50 ± 0.28 i |
| 6-Hydroxyflavanone (3) | 53.55 ± 0.45 b | 69.21 ± 0.58 b |
| (+)-Dihydrokaempferol (4) | 45.35 ± 0.60 a | 55.41 ± 0.33 a |
| 3,4-Dihydroxybenzoic acid (5) | 64.54 ± 0.65 d | 84.66 ± 0.90 c |
| Taraxerol (6) | 255.32 ± 0.15 g | 276.56 ± 0.56 f |
| Taraxerone (7) | 75.45 ± 0.44 e | 95.64 ± 0.45 d |
| Lupeol acetate (8) | 155.66 ± 0.51 f | 139.99 ± 0.33 e |
| Kojic acid * | 58.53 ± 0.35 c | 53.43 ± 0.38 a |
| α-Arbutin * | 353.53 ± 0.55 i | 365.93 ± 0.45 h |
| Compound | IC50 (μM) | FRAP (μM) | |
|---|---|---|---|
| DPPH | ABTS | ||
| Taraxerol methyl ether (1) | 77.31 ± 0.60 f | 520.22 ± 0.30 f | 1.31 ± 0.16 a |
| Spinasterol (2) | 93.10 ± 0.84 h | 921.21 ± 0.42 i | 1.54 ± 0.21 a |
| 6-Hydroxyflavanone (3) | 3.21 ± 0.70 b | 225.53 ± 0.95 c | 4.12 ± 0.12 c |
| (+)-Dihydrokaempferol (4) | 2.21 ± 0.77 a | 214.83 ± 0.51 b | 6.23 ± 0.10 d |
| 3,4-Dihydroxybenzoic acid (5) | 4.71 ± 0.10 c | 290.14 ± 0.95 d | 3.00 ± 0.40 b |
| Taraxerol (6) | 16.28 ± 0.33 e | 630.84 ± 0.54 g | 1.46 ± 0.11 a |
| Taraxerone (7) | 10.20 ± 0.40 d | 334.83 ± 0.99 e | 1.12 ± 0.13 a |
| Lupeol acetate (8) | 87.10 ± 0.31 g | 669.62 ± 0.42 h | 1.28 ± 0.30 a |
| Trolox * | 1.92 ± 0.22 a | 188.39 ± 0.43 a | 6.10 ± 0.28 d |
| Compound | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| BT474 | Chago-K1 | HepG2 | KATO-III | SW620 | WI-38 | |
| Taraxerol methyl ether (1) | 184.95 ± 1.61 g | >227.07 h | >227.07 g | >227.07 g | >227.07 f | >227.07 d |
| Spinasterol (2) | 9.16 ± 1.97 b | 16.53 ± 2.84 c | 10.87 ± 1.12 b | 13.73 ± 3.69 b | 33.03 ± 2.50 b | 9.85 ± 1.90 a |
| 6-Hydroxyflavanone (3) | 86.16 ± 0.45 f | 57.73 ± 1.08 e | 65.76 ± 2.37 e | 88.78 ± 3.70 e | 82.79 ± 1.33 d | >416.22 g |
| (+)-Dihydrokaempferol (4) | 11.66 ± 0.42 c | 12.32 ± 0.73 b | 13.67 ± 0.38 c | 39.79 ± 0.38 d | 41.11 ± 1.08 c | >346.92 h |
| 3,4-Dihydroxybenzoic acid (5) | 85.21 ± 3.96 f | 79.22 ± 4.02 f | 364.72 ± 2.27 i | 507.53 ± 4.61 i | 591.36 ± 0.71 i | >648.85 i |
| Taraxerol (6) | >235.45 h | >235.45 i | >235.45 h | >235.45 h | >235.45 h | >235.45 f |
| Taraxerone (7) | 19.24 ± 0.40 d | 26.75 ± 0.97 d | 20.41 ± 1.43 d | 26.49 ± 0.57 c | >234.34 g | >234.34 e |
| Lupeol acetate (8) | 60.20 ± 0.90 e | 199.87 ± 0.30 g | >213.33 f | 136.68 ± 0.66 f | 182.67 ± 1.51 e | >213.33 c |
| Doxorubicin * | 1.21 ± 0.20 a | 1.58 ± 0.40 a | 2.70 ± 0.83 a | 1.78 ± 0.83 a | 1.82 ± 0.39 a | >183.99 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24152798
Chunhakant S, Chaicharoenpong C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules. 2019; 24(15):2798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24152798
Chicago/Turabian StyleChunhakant, Sutthiduean, and Chanya Chaicharoenpong. 2019. "Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark" Molecules 24, no. 15: 2798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24152798