Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects
Abstract
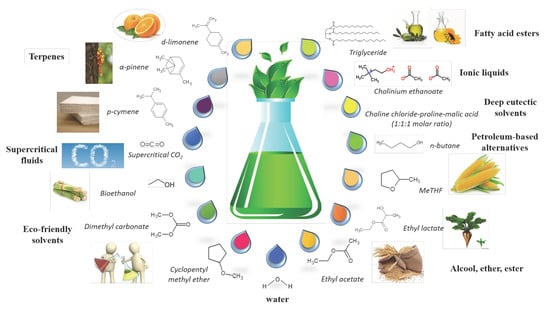
:1. Introduction
2. Solvent-Free Extraction
3. Water as Green Solvent
4. Green Solvents from Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs) to Natural Deep Eutectic Solvents (NADESs)
5. Biobased-Solvents
6. Liquefied Gases: From Supercritical Fluid to Liquefied Gas Extraction
- Non-isobaric: In non-isobaric conditions, the liquefied gas is flowed through the raw material using a circulating pump, then evaporated by expansion and finally liquefied by a compressor. This way of doing is very similar to the working principle of cooling units. Such equipment allows precise control of the flow rate and working pressure. In addition, the solvent can be driven “up-flow” to ensure a maximum solid/liquid contact. However, pumps and compressors are expensive equipment that requires frequent maintenance operations, especially with liquefied gases. Moreover, the size of compressors is typically a limiting factor for large industrial applications, especially in the case of flammable gases.
- Isobaric: Recirculation of solvent can also be achieved without a pump or compressor by using isobaric conditions. In this case, the system always stays at liquid/vapor equilibrium, the operating pressure is equal to the vapor pressure of the solvent. In that case, the liquefied solvent is transferred from a vessel to another by the only help of gravity. The liquefied gas is then evaporated in the boiler under the same pressure (isobaric mode) and the vapors naturally rise to the condenser for solvent regeneration. The absence of mechanical equipment leads to lower energy consumption and maintenance cost. However, the flow-rate only depends on the performance of the boiler and condenser that require careful design and monitoring.
7. Intensification as a Key for Industrial Success Stories of Green Solvents
8. Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Choi, Y.H.; Verpoorte, R. Metabolomics: What you see is what you extract. Phytochem. Anal. 2014, 25, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Kerton, F.M.; Mariotte, R. Alternative Solvents for Green Chemistry, 2nd ed.; Royal Society of Chemistry: Croydon, UK, 2013; pp. 1–325. [Google Scholar]
- Henderson, R.K.; Jimenez-Gonzalez, C.; Constable, D.J.C.; Alston, S.A.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.S.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.W.; Letetsu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Cruciani, P.; Hosek, P. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters—A User’s Handbook; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Klamt, A.; Eckert, F.; Arlt, W. COSMO-RS: An Alternative to Simulation for Calculating Thermodynamic Properties of Liquid Mixtures. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 101–122. [Google Scholar] [CrossRef] [Green Version]
- Bergez-Lacoste, M.; Thiebaud-roux, S.; De Caro, P.; Fabre, J. From chemical platform molecules to new biosolvents: Design engineering as a substitution methodology. Biofuels Bioprod. Biorefin. 2014, 438–451. [Google Scholar] [CrossRef]
- Chemat, F.; Smadja, J.; Lucchesie, M.E. Solvent Free Microwave Extraction of Volatile Natural Compound. US Patent 0187340 A1, 30 September 2004. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=US&NR=2004187340A1&KC=A1&FT=D&ND=3&date=20040930&DB=EPODOC&locale=fr_EP# (accessed on 17 August 2019).
- Chemat, F.; Abert Vian, M.; Visinoni, F. Microwave Hydro-diffusion for Isolation of Natural Products. European Patent EP 1 955 749 A1, 12 August 2008. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=10&ND=4&adjacent=true&locale=fr_EP&FT=D&date=20100528&CC=EP&NR=1955749A1&KC=A1# (accessed on 17 August 2019).
- Monrad, J.K.; Suarez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of anthocyanins and flavan-3-ols from red grape pomace continuously by coupling hot water extraction with a modified expeller. Food Res. Int. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Kips, L.D.; De Paepe, L.; Van Meulebroek, C.; Van Poucke, B.; Van Droogenbroeck, B. A novel spiral-filter press for tomato processing: Process impact on phenolic compounds, carotenoids and ascorbic acid content. J. Food Eng. 2017, 213, 27–37. [Google Scholar] [CrossRef]
- Sayasoonthorn, S.; Kaewrueng, S.; Patharasathapornkul, P. Rice bran oil extraction by screw press method: Optimum operating settings, oil extraction level and press cake appearance. Rice Sci. 2012, 19, 75–78. [Google Scholar] [CrossRef]
- Labuckas, D.; Maestri, D.; Lamarque, A. Effect of different oil extraction methods on proximate composition and protein characteristics of walnut Juglans regia L. flour. LWT Food Sci. Technol. 2014, 1, 794–799. [Google Scholar] [CrossRef]
- Louati, I.; Bahloul, N.; Besombes, C.; Allaf, K.; Kechaou, N. Instant controlled pressure-drop as texturing pretreatment for intensifying both final drying stage and extraction of phenolic compounds to valorize orange industry by-products (Citrus sinensis L.). Food Bioprod. Process. 2019, 114, 85–94. [Google Scholar] [CrossRef]
- Rashidi, S.; Eikani, M.; Ardjmand, M. Extraction of Hyssopus officinalis L. essential oil using instant controlled pressure drop process. J. Chromatogr. A 2018, 1579, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Benamor, B.; Allaf, K. Impact of texturing using instant pressure drop treatment prior to solvent extraction of anthocyanins from Malaysian Roselle (Hibiscus sabdariffa). Food Chem. 2009, 115, 820–825. [Google Scholar] [CrossRef]
- Benamor, B.; Lamy, C.; Andre, P.; Allaf, K. Effect of instant controlled pressure drop treatments on the oligosaccharides extractability and microstructure of Tephrosia purpurea seeds. J. Chromatogr. A 2008, 1213, 118–124. [Google Scholar]
- Wei, Z.F.; Zhao, R.N.; Dong, L.J.; Zhao, X.Y.; Zhang, L.J. Dual-cooled solvent-free microwave extraction of Salvia officinalis L. essential oil and evaluation of its antimicrobial activity. Ind. Crops Prod. 2018, 120, 71–76. [Google Scholar] [CrossRef]
- Turk, M.; Perino, S.; Cendres, A.; Petitcolas, E.; Soubrat, T.; Chemat, F. Alternative process for strawberry juice processing: Microwave hydrodiffusion and gravity. LWT Food Sci. Technol 2017, 84, 626–633. [Google Scholar] [CrossRef]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to pilot scale: Microwave extraction for polyphenols lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef]
- Zill-e, H.; Abert Vian, M.; Fabiano-Tixier, A.S.; El Maataoui, M.; Dangles, O.; Chemat, F. A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties. Food Chem. 2011, 127, 1472–1480. [Google Scholar] [CrossRef]
- Pataro, D.C.; Bakar Siddique, M.A.; Falcone, M.; Ferrari, G. Improved extractability of carotenoids from tomato peels as side benefits of PEF treatment of tomato fruit for more energy-efficient steam-assisted peeling. J. Food Eng. 2018, 233, 65–73. [Google Scholar] [CrossRef]
- Puertolas, E.; Cregenzan, O.; Luengo, E.; Alvarez, I.; Raso, J. Pulsed-electric-field assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoiselle, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Marthe, S.J.; Jadhav, S.B.; Bankar, S.B.; Dubey, K.K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [Green Version]
- Catlkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Verdasco-Martin, C.; Diaz-Lozano, A.; Otero, C. Advantageous enzyme selective extraction process of essential spirulina oil. Catal. Today 2019. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R.; Najafi, M.N. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innov. Food Sci. Emerg. Technol. 2016, 36, 234–244. [Google Scholar] [CrossRef]
- Yulianto, M.E.; Kusumo, P.; Hartati, I.; Wahyuningsih, A. Subcritical water extraction of gingerol from Zingiber officinale. Rasayan, J. Chem. 2017, 10, 734–738. [Google Scholar]
- Kiamahalleh, M.V.; Najafpour-Darzi, G.; Rahimnejad, M.; Moghadamnia, A.A. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J. Chromatogr. A 2016, B1022, 191–198. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Gogus, F.; Lewis, A.C. Subcritical water extraction of essential oils from Thymbra spicate. Food Chem. 2003, 82, 381–386. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Marostica Junior, M.R.; Rostagno, M.A.; Martinez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Su, D.L.; Li, P.J.; Quek, S.Y.; Huang, Z.Q.; Yuan, Y.J.; Li, G.Y.; Shan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Neuberg, C. Hydrotropic phenomena. Biochemistry 1916, 76, 107–176. [Google Scholar]
- Kunz, W.; Holmberg, K.; Zemb, T. Hydrotropes. Cur. Opin. Colloid Interface Sci. 2016, 22, 99–107. [Google Scholar] [CrossRef]
- Dandekar, D.V.; Jayaprakasha, G.K.; Patil, B.S. Hydrotropic extraction of bioactive limonin from sour orange (Citrus aurantium L.) seeds. Food Chem. 2008, 109, 515–520. [Google Scholar] [CrossRef]
- Desai, M.A.; Parikh, J. Hydrotropic extraction of citral from cymbopogon flexuosus. Ind. Eng. Chem. Res. 2012, 51, 3750–3757. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Tian, M.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B 2012, 904, 1–21. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.Á.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TRaC Trend Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyana, M.; Hayyan, A.; Wong, W.F.; Hashima, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and Bioengineering-Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Dai, Y.; Spronsen, J.V.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products. Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019. [Google Scholar] [CrossRef]
- Choi, Y.H.; Spronsen, J.V.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2017, 33, 1–6. [Google Scholar] [CrossRef]
- Verpoorte, R. Secondary metabolism. In Metabolic Engineering of Plant Secondary Metabolism; Verpoorte, R., Alfermann, A.W., Eds.; Kluwer Academic Publidhers: Dordrecht, The Netherlands, 2000; pp. 1–29. [Google Scholar]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Fernández, M.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta. 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, G.; Peng, G.; Li, J.; Chen, J. Application of Ionic Liquid-Based Ultrasound-Assisted Extraction of Five Phenolic Compounds from Fig (Ficus carica L.) for HPLC-UV. Food Anal. Methods 2015, 8, 1673–1681. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Lai, C.J.S.; OuYang, H.; He, M.Z.; Feng, Y. Ionic liquid-based ultrasound-assisted extraction and aqueous two-phase system for analysis of caffeoylquinic acids from Flos Lonicerae japonicae. J. Pharm. Biomed. Anal. 2016, 120, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.; Xua, L.; Ma, J.; Li, J.; Maa, R.; Sun, K.; Wang, Z.; Zhang, H. Ionic liquid-based salt-induced liquid-liquid extraction of polyphenols and anthraquinones in Polygonum cuspidatum. J. Pharm. Biomed. Anal. 2019, 163, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.A.; Vijayaraghavan, R.; MacFarlane, D.R. Distillable ionic liquid extraction of tannins from plant materials. Green Chem. 2010, 12, 1023–1028. [Google Scholar] [CrossRef]
- Wang, X.H.; Cai, C.; Li, X.M. Optimal Extraction of Gallic Acid from Suaeda glauca Bge. Leaves and Enhanced Efficiency by Ionic Liquids. Int. J. Chem. Eng. 2016, 1–9. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, M.; Tan, T.; Yan, A.; Guo, L.; Jiang, K.; Tan, C.; Wan, Y. Deep eutectic solvents used as extraction solvent for the determination of flavonoids from Camellia oleifera flowers by high-performance liquid chromatography. Phytochem. Anal. 2018, 29, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Thakker, M.R.; Parikh, J.K.; Desai, M.A. Synergism between ionic liquid and ultrasound forgreener extraction of geraniol: Optimization using different statistical tools, comparison and prediction. Chem. Eng. Res. Des. 2018, 134, 162–171. [Google Scholar] [CrossRef]
- Li, M.; Dong, S.; Li, N.; Tang, H.; Zheng, J. Magnetic Fe3 O4 carbon aerogel and ionic liquid composite films as an electrochemical interface for accelerated electrochemistry of glucose oxidase and myoglobin. RSC Adv. 2015, 5, 14704–14711. [Google Scholar] [CrossRef]
- Rodrigues, R.D.; De Castro, F.C.; De Santiago-Aguiar, R.S.; Rocha, M.V.P. Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res. 2018, 31, 454–462. [Google Scholar] [CrossRef]
- Fan, Y.; Xua, C.; Lic, J.; Zhang, L.; Yanga, L.; Zhoua, Z.; Zhua, Y.; Zhao, D. Ionic liquid-based microwave-assisted extraction of verbascoside from Rehmannia root. Ind. Crops Prod. 2018, 124, 59–65. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Murador, D.C.; De Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; De Rosso, V.V. Bioavailability and biological effects of bioactive compounds extracted with natural deep eutectic solvents and ionic liquids: Advantages over conventional organic solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Fernández, M.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Yoo, E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Yang, M.; Cao, J.; Cao, F.; Lu, C.; Su, E. Efficient Extraction of Bioactive Flavonoids from Ginkgo biloba Leaves Using Deep Eutectic Solvent/Water Mixture as Green Media. Chem. Biochem. Eng. Q. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from PollenTyphae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, M.; Guo, H.; Xu, J.; Ye, J.; Zhao, J.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media for the extraction of flavonoids from Radix scutellariae. New J. Chem. 2019, 43, 644–650. [Google Scholar] [CrossRef]
- Đorđević, B.S.; Todorović, Z.B.; Troter, D.Z.; Stanojević, L.P.; Veljković, V.B. The extraction of quercetin from waste onion (Allium cepa L.) tunic by the aqueous solutions of different deep eutectic solvents. Adv. Technol. 2018, 7, 5–10. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Hu, H.; Wan, J.; Li, P. Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations. Pharmaceutics 2019, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Bi, W.; Zhang, H.; Row, K.H. Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia 2014, 77, 373–377. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-designed hydrophobic deep eutectic solvents as green and efficient media for the extraction of artemisinin from Artemisia annua leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.; Row, K.H. A green deep eutectic solvent-based ultrasound-assisted method to extract astaxanthin from shrimp byproducts. Anal. Lett. 2014, 47, 742–749. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brncić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Nie, J.; Yu, G.; Song, Z.; Wang, X.; Li, Z.; She, Y.; Lee, M. Microwave-assisted deep eutectic solvent extraction coupled with headspace solid-phase microextraction followed by GC-MS for the analysis of volatile compounds from tobacco. Anal. Methods 2017, 9, 856–863. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, L.-J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.-H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Gómez, A.V.; Tadinia, C.C.; Biswas, A.; Buttrum, M.; Kim, S.; Boddu, V.M.; Cheng, H.N. Microwave-assisted extraction of soluble sugars from banana puree with natural deep eutectic solvents (NADES). LWT J. Food Sci. Technol. 2019, 107, 79–88. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Deep eutectic solvents (DES) mediated extraction of pectin from Averrhoa bilimbi: Optimization and characterization studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hadi, N.M.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of choline-based deep eutectic solvents in the extraction of tocols from crude palm oil. J. Am. Oil Chem. Soc. 2015, 92, 1709–1716. [Google Scholar] [CrossRef]
- Bai, C.; Wei, Q.; Ren, X. Selective extraction of collagen peptides with High purity from cod skins by deep eutectic solvents (DESs). ACS Sustain. Chem. Eng. 2017, 5, 7220–7227. [Google Scholar] [CrossRef]
- Lavaud, A.; Laguerre, M.; Bitric, S.; Fabiano Tixier, A.-S.; Roller, M.; Chemat, F. International. Patent WO 2016/162703 Al, 10 April 2015. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=4&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20180406&CC=CN&NR=107889468A&KC=A# (accessed on 17 August 2019).
- Makkeri, C.; Moser, P.; Chemat, F.; Perino, S. Utilisation de L’eau de Coco Comme solvant D’extraction. Fr Patent FR 3061416 A1, 29 December 2016. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=5&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20180705&CC=WO&NR=2018122514A1&KC=A1# (accessed on 17 August 2019).
- Calvo, F.G.; María, F.; Monteagudo, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Lomba, L.; Zuriaga, E.; Giner, B. Solvents derived from biomass and their potential as green solvents. Curr. Opin. Green Sustain. Chem. 2019, 18, 51–56. [Google Scholar] [CrossRef]
- Khoo, H.H.; Wong, L.L.; Tan, J.; Isoni, V.; Sharratt, P. Synthesis of 2-methyl tetrahydrofuran from various lignocellulosic feedstocks: Sustainability assessment via LCA. Res. Conserv. Recycl. 2015, 95, 174–182. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process Res. Dev. 2007, 112, 251–258. [Google Scholar] [CrossRef]
- Wu, W.; Maravelias, C.T. Biotechnology for Biofuels Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnol. Biofuels 2018, 11, 294. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Chaabani, E.; Abert Vian, M.; Dakhlaoui, S.; Bourgou, S.; Chemat, F.; Ksouri, R. Pistacia lentiscus L. edible oil: Green extraction with bio-based solvents, metabolite profiling and in vitro anti-inflammatory activity. OCL 2019, 26, 25. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Rezaei, K. Pressurized limonene as an alternative bio-solvent for the extraction of lipids from marine microorganisms. J. Supercrit. Fluids 2014, 92, 1–7. [Google Scholar] [Green Version]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Extraction of peroxidase from bitter gourd (Momordica charantia) by three-phase partitioning with dimethyl carbonate (DMC) as organic phase. Process Biochem. 2017, 61, 195–201. [Google Scholar] [CrossRef]
- Popov, S.A.; Sheremet, O.P.; Kornaukhova, L.M.; Grazhdannikov, A.E.; Shults, E.E. An approach to effective green extraction of triterpenoids from outer birch bark using ethyl acetate with extractant recycle. Ind. Crops Prod. 2017, 102, 122–132. [Google Scholar] [CrossRef]
- Ibrahim, A.P.; Omilakin, R.O.; Betiku, E. Optimization of microwave-assisted solvent extraction of non-edible sandbox (Hura crepitans) seed oil: A potential biodiesel feedstock. Renew. Energy 2019, 141, 349–358. [Google Scholar] [CrossRef]
- Antonio, A.; Archivio, D.; Anna, M.; Ruggieri, F. Extraction of curcuminoids by using ethyl lactate and its optimization by response surface methodology. J. Pharm. Biomed. Anal. 2018, 149, 89–95. [Google Scholar]
- Bermejo, D.V.; Mendiola, J.A.; Ibá, E.; Reglero, G.; Fornari, T. Pressurized liquid extraction of caffeine and catechins from green tea leaves using ethyl lactate, water and ethyl lactate + water mixtures. Food Bioprod. Process. 2015, 6, 106–112. [Google Scholar] [CrossRef]
- Taylor, P.; Bertouche, S.; Tomao, V.; Hellal, A.; Boutekedjiret, C.; Chemat, F. First approach on edible oil determination in oilseeds products using alpha-pinene. J. Essent. Oil Res. 2013, 25, 439–443. [Google Scholar]
- Bertouche, S.; Tomao, V.; Ruiz, K.; Hellal, A.; Boutekedjiret, C.; Chemat, F. First approach on moisture determination in food products using alpha-pinene as an alternative solvent for Dean–Stark distillation. Food Chem. 2012, 134, 602–605. [Google Scholar] [CrossRef]
- Badens, E. Mise en forme de principes actifs pharmaceutiques en phase supercritique. Techniques de l’Ingénieur, TIB611DUO, 27 March 2012.
- Lumia, G. Extraction par fluides supercritiques. In Eco-Extraction du Végétal; Chemat, F., Ed.; Dunod: Paris, France, 2011; pp. 231–258. [Google Scholar]
- Samppatrao, D.M.; Dhindra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Sánchez-Camargo, A.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical Fluid Extraction of Essential Oils. TrAC 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Akgun, M.; Akgun, N.A.; Dincer, S. Phase behaviour of essential oil components in supercritical carbon dioxide. J. Supercrit. Fluids 1999, 15, 117–125. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2012, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernandez, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltran, J.A. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Suhajda, Á.; Héthelyi, É. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res. Int. 2005, 38, 51–57. [Google Scholar] [CrossRef]
- Haque Akanda, M.J.; Islam Sarker, M.Z.; Ferdosh, S.; Abdul Manap, M.Y.; Nik Ab Rahman, N.N.; Ab Kadir, M.O. Applications of Supercritical Fluid Extraction (SFE) of Palm Oil and Oil from Natural Sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgina, U.; Calimli, A.; Uysal, B.Z. Supercritical Fluid Extraction of Jojoba Oil. J. Am. Oil Chem. Soc. 2004, 81, 293–296. [Google Scholar] [CrossRef]
- Kumar Saini, R.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimization and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Novello, Z.; Scapinello, J.; Magro, J.D.; Zin, G.; Luccio, M.D.; Tres, M.V.; Oliveira, J.V. Extraction, chemical characterization and antioxidant activity of andiroba seeds oil obtained from pressurized n-butane. Ind. Crops Prod. 2015, 76, 697–701. [Google Scholar] [CrossRef]
- Rapinel, V.; Rombaut, N.; Rakotomanomana, N.; Vallageas, A.; Cravotto, G.; Chemat, F. An original approach for lipophilic natural products extraction: Use of liquefied n -butane as alternative solvent to n -hexane. LWT Food Sci. Technol. 2017, 85, 524–533. [Google Scholar] [CrossRef]
- Sparks, D.; Hernandez, R.; Zappi, M.; Blackwell, D.; Fleming, T. Extraction of rice bran oil using supercritical carbon dioxide and propane. J. Am. Oil Chem. Soc. 2006, 83, 885–891. [Google Scholar] [CrossRef]
- Da Silva, C.M.; Zanqui, A.B.; Gohara, A.K.; de Souza, A.H.; Cardozo-Filho, L.; Visentainer, J.V.; Rovigatti Chiavelli, L.; Bittencourt, P.R.; da Silva, E.A.; Matsushita, M. Compressed n-propane extraction of lipids and bioactive compounds from Perilla (Perilla frutescens). J. Supercrit. Fluids 2015, 102, 1–8. [Google Scholar] [CrossRef]
- Corso, M.P.; Fagundes-Klen, M.R.; Silva, E.A.; Cardozo, L.; Santos, J.N.; Freitas, L.S.; Dariva, C. Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J. Supercrit. Fluids 2010, 52, 56–61. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Goto, M.; Makino, H. Energy-saving lipid extraction from wet Euglena gracilis by the low-boiling point solvent dimethyl ether. Energies 2015, 8, 610–620. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Yoshimura, T.; Okada, L. Wet extraction of hydrocarbons from Botryococcus braunii by dimethyl ether as compared with dry extraction by hexane. Fuel 2013, 105, 535–539. [Google Scholar] [CrossRef]
- Hoshino, R.; Ogawa, M.; Murakami, K.; Wahyudiono, K.H.; Goto, M. Extraction of lipids from wet Arthrospira platensis by liquefied dimethyl ether. Solvent Extr. Res. Dev. Jpn. 2017, 24, 47–60. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.; De Oliveira, J.S.; Côcco, L.C.; Da Luz Costa, J.; De Carvalho, J.C.; Soccol, C.R. Liquefied gas extraction: A new method for the recovery of terpenoids from agroindustrial and forest wastes. J. Supercrit. Fluids 2016, 110, 97–102. [Google Scholar] [CrossRef]
- Nenov, N.; Gochev, V.; Girova, T.; Stoilova, I.; Atanasova, T.; Stanchev, V.; Stoyanova, A. Low temperature extraction of essential oil-bearing plants by liquefied gases. 6. Barks from cinnamon (Cinnamomum zeylanicum Nees). J. Essent. Oil Bear. Plants 2011, 14, 67–75. [Google Scholar] [CrossRef]
- Hoshino, R.; Machmudah, S.; Kanda, H.; Goto, M. Simultaneous extraction of water and essential oils from citrus leaves and peels using liquefied dimethyl ether. J. Nutr. Food Sci. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Zanqui, A.B.; de Morais, D.R.; da Silva, C.M.; Santos, J.M.; Chiavelli, L.U.R.; Bittencourt, P.R.S.; Eberlin, M.N.; Visentainer, J.V.; Cardozo-Filho, L.; Matsushitaa, M. Subcritical Extraction of Salvia hispanica L. Oil with N-Propane: Composition, Purity and Oxidation Stability as Compared to the Oils Obtained by Conventional Solvent Extraction Methods. J. Braz. Chem. Soc. 2015, 26, 282–289. [Google Scholar]
- Goto, M.; Kanda, H.; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Romdhane, M.; Gourdon, C. Investigation in solid—Liquid extraction: Influence of ultrasound. Chem. Eng. J. 2002, 87, 11–19. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Turk, M.; Fabiano-tixier, A.S.; Petitcolas, E.; Robinet, P.; Imbert, R.; El Maataoui, M.; Chemat, F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018, 42, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Veillet, S.; Tomao, V.; Chemat, F. Ultrasound assisted maceration: An original procedure for direct aromatisation of olive oil with basil. Food Chem. 2010, 123, 905–911. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Ultrasonics Sonochemistry Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Yara-Varon, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-tixier, A.S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Maximo, G.J.; Santos, R.J.B.N.; Lopes-Da-Silva, J.A.; Costa, M.C.; Meirelles, A.J.A.; Coutinho, J.A.P. Lipidic protic ionic liquid crystals. ACS Sustain. Chem. Eng. 2014, 2, 672–682. [Google Scholar] [CrossRef]
- Liu, Q.P.; Hou, X.D.; Li, N.; Zong, M.H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Rao, X.; Zhang, J.; Zheng, J.; Song, Z.; Shang, S. Chiral ionic liquid crystals with a bulky rigid core from renewable camphor sulfonic acid. RSC Adv. 2014, 4, 25334–25340. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Gurbisz, M.; Ghavre, M.; Ferreira, F.M.M.; Gonçalves, F.; Beadham, I.; Quilty, B.; Coutinho, J.A.P.; Gathergood, N. Imidazolium and pyridinium ionic liquids from mandelic acid derivatives: Synthesis and bacteria and algae toxicity evaluation. ACS Sustain. Chem. Eng. 2013, 1, 393–402. [Google Scholar] [CrossRef]
- Álvarez, V.H.; Mattedi, S.; Martin-Pastor, M.; Aznar, M.; Iglesias, M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluids Phase Equilib. 2010, 299, 42–50. [Google Scholar] [CrossRef]
- Ni, X.; Xing, H.; Yang, Q.; Wang, J.; Su, B.; Bao, Z.; Yang, Y.; Ren, Q. Selective liquid-liquid extraction of natural phenolic compounds using amino acid ionic liquids: A case of α-tocopherol and methyl linoleate separation. Ind. Eng. Chem. Res. 2012, 51, 6480–6488. [Google Scholar] [CrossRef]
- Hou, X.D.; Li, N.; Zong, M.H. Facile and simple pretreatment of sugar cane bagasse without size reduction using renewable ionic liquids water mixtures. ACS Sustain. Chem. Eng. 2013, 1, 519–526. [Google Scholar] [CrossRef]
- Hou, X.D.; Li, N.; Zong, M.H. Renewable bio ionic liquids-water mixtures-mediated selective removal of lignin from rice straw: Visualization of changes in composition and cell wall structure. Biotechnol. Bioeng. 2013, 110, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.D.; Kim, H.J.; Sun, J.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Moriel, P.; García-Suárez, E.J.; Martínez, M.; García, A.B.; Montes-Morán, M.A.; Calvino-Casilda, V.; Bañares, M.A. Synthesis, characterization, and catalytic activity of ionic liquids based on biosources. Tetrahedron Lett. 2010, 51, 4877–4881. [Google Scholar] [CrossRef]
- Aroso, I.M.; Craveiro, R.; Rocha, A.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behaviorof deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Shamshina, J.; Cojocaru, O.A.; Janikowski, J.; MacFarlane, D.R.; Davis, J.H.; Rogers, R.D. Simultaneous membrane transport oftwo active pharmaceutical ingredients by charge assisted hydrogen bondcomplex formation. Chem. Sci. 2014, 5, 3449–3456. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients andtherapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of functional therapeutic deep eutectic solvents based on choline chloride and ascorbic acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Bado-Nilles, A.; Diallo, A.-O.; Marlair, G.; Pandard, P.; Chabot, L.; Geffard, A.; Len, C.; Porcher, J.-M.; Sanchez, W. Coupling of OECD standardized test and immunomarkers to select the most environmentally benign ionic liquids option—Towards an innovative “safety by design” approach. J. Hazard. Mater. 2015, 283, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.-O.; Morgan, A.B.; Len, C.; Marlair, G. An innovative experimental approach aiming to understand and quantify the actual fire hazards of ionic liquids. Energy Environ. Sci. 2013, 6, 699. [Google Scholar] [CrossRef]
- Diallo, A.-O.; Fayet, G.; Len, C.; Marlair, G. Evaluation of Heats of Combustion of Ionic Liquids through Use of Existing and Purpose-Built Models. Ind. Eng. Chem. Res. 2012, 51, 3149–3156. [Google Scholar] [CrossRef]
- Ali, M.C.; Liu, R.; Chen, J.; Cai, T.; Zhang, H.; Li, Z.; Zhai, H.; Qiu, H. New deep eutectic solvents composed of crown ether, hydroxide and polyethylene glycol for extraction of non-basic N-compounds. Chin. Chem. Lett. 2019, 30, 871–874. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, M.; Tan, T.; Chen, J.; Li, Z.; Zhang, Q.; Qiu, H. Deep eutectic solvents as novel extraction media for phenolic compounds from model oil. Chem. Commun. 2014, 50, 11749–11752. [Google Scholar] [CrossRef]






| Material | Analyte | Process/Conditions | Analysis | Ref |
|---|---|---|---|---|
| Red grape | Anthocyanins | Expeller | UV-visible, HPLC | [11] |
| Tomato | Carotenoids | Spiral-filter press | UPLC-MS-Ms | [12] |
| Rice bran | Vegetable oil | Screw press | GC-MS | [13] |
| Walnut floor | Vegetable oil | Hydraulic press | UV-visible | [14] |
| Orange peel | Polyphenols | DIC: 0.6 MPa, 20 s, 6 cycles | HPLC-DAD | [15] |
| Hyssorpus | Essential oil | DIC: 1 MPa, 100 s, 12 cycles | GC-FID, GC-MS | [16] |
| Roselle | Anthocyanins | DIC: 0.18 MPa, 20 s, 1 cycle | UV-Visible, HPLC | [17] |
| Tephrosia seeds | Ciceritol | DIC: 0.6 MPa, 240 s, 1 cycle | HPLC-DAD | [18] |
| Salvia officinalis | Essential oil | SFME: 650 W, 35 min | GC-MS/GC-FID | [19] |
| Strawberry | Aromatic compounds | MHG, 1000 W/kg, 30 min. | GC-MS | [20] |
| Lettuce Onions | Polyphenols | SFME: P.atm, 1 W/g, 15–50 min MHG, 500 g, P(atm) 300–900 W, T = 5–70 min | HPLC-DAD | [21,22] |
| Tomato | Carotenoids | PEF: 0.5 kV/cm, 1kJ/kg, 60 °C, water | HPLC-DAD | [23] |
| Purple-fleshed potato | Anthocyanins | PEF: 3.4 kV/cm, 35 pulses, 40 °C, ethanol | HPLC-DAD | [24] |
| Grape seeds | Polyphenols | PEF: 5 kV/cm, 1–5 pulses, 30% ethanol | UV-Visible | [25] |
| Properties | ILs | DESs Including NADESs |
|---|---|---|
| Intermolecular force | Ionic bonding | Hydrogen bonding |
| Melting point | Below 100 °C | |
| Vapor pressure | Low | |
| Viscosity | High viscosity, Positive linear correlation with temperature | |
| Dissolving ability | A broad range of polar and nonpolar molecules | |
| Cytotoxicity | Positive for many | Hard to detect |
| Material | Method | Analyte | ILs Composition | Ref. |
|---|---|---|---|---|
| Ficus carica L. | UAE | Phenolic compounds | [C4MIM][PF6](water) | [57] |
| Eucalyptus leaves | MAE | [HO3S(CH2)4MIM]HSO4 (water) | [58] | |
| Lonicerae Japonicae Flos | UAE | [C4MIM]Br (water) | [59] | |
| Polygonum cuspidatum | LLE | Polyphenols and anthraquinones | C6H5Na3O2 (water); (NH4)2SO4; NaHCO3 | [60] |
| Catechu and myrobolan | SPME | Tannin | DIMCARB | [61] |
| Suaeda glauca Bge. Leaves | UAE | Gallic acid | [C6MIM]Cl (ethanol) | [62] |
| Lotus leaves | MAE | nornuciferine | [HMIM][Br] | [63] |
| Palmarosa leaves | UAE | Geraniol | DIL-2 | [64] |
| Farfarae Flos | Distillation | Essential oils | [C4MIM] [CH3COO] (water) | [65] |
| Spirulina platensis | UAE | Phycobiliproteins | 2-HEAA; [BMIM][Cl] | [66] |
| Rehmannia root | MAE | Verbascoside | [BMIM]Cl | [67] |
| Material | Method | Analyte | DESs/NADESs Composition | Ref. |
|---|---|---|---|---|
| Grape skin | UAE, MAE | Phenolic Compounds | ChCl:OA, water 25% | [71] |
| Onion, olive, pear | UAE | LA:Glu; CA:Glu; Fru:CA | [72] | |
| Olive pomace | MAE; UAE | ChCl:CA; ChCl:LA; ChCl:Gly | [73] | |
| Spent coffee | UAE | 1,6-HD:ChCl (7:1) | [74] | |
| Orange peel waste | SLE | ChCl:EG (1:4), water 10% | [75] | |
| Ginkgo biloba | Stirring | Flavonoids | ChCl:La, water 40% (w/w) | [76] |
| PollenTyphae | UAE | ChCl:1,2-PD (1:4), water 30% | [77] | |
| Radix scutellariae | UAE | Pro:Gly(1:4) | [78] | |
| Allium cepa L. | SLE | Quercetin | ChCl:U | [79] |
| Jinqi Jiangtang Preparations | UAE | Phenolic acids and alkaloids | ChCl:La (1:2); ChCl:Gly (1:2); ChCl:Glu (1:1); Pro:MA (1:1) | [80] |
| Chamaecyparis | HS-SME | Terpenoids | ChCl:EG | [81] |
| Artemisia annua | UAE | Artemisinin | MTA-Ch:B (1:4) | [82] |
| Shrimp by-products | UAE | Astaxanthin | ChCl:EG; ChCl:Gly; ChCl:1,2-BD; ChCl:1,3-BD; ChCl:1,4-BD | [83] |
| Catharanthus roseus | Heating and stirring | Anthocyanins | ChCl:1,2-PD; LA:Glu; Pro:MA; ChCl:MA; ChCl:Glu; Glu:Fru:Suc | [84] |
| Wine lees | UAE | ChCl:MA | [85] | |
| Vanilla pods | SLE | Vanillin | 14 NADESs/MA:Glu:water (1:1:6); MA:Fru:Glu:water (1:1:1:9) | [52] |
| Nicotiana tabacum L. | MAE | Volatile compounds | ChCl:Gly; ChCl:U; Cap:U | [86] |
| Caulis sinomenii, Coptis chinensis, Stephania tetrandra, Sophora flavescens | UAE | Morphinane, protoberberine, bisbenzylisoquinoline and indole alkaloids | 75 types of binary or ternary DESs/ChCl-LA 1:2, 30% water | [87] |
| Banana puree | MAE | Soluble sugars | MA:BA:water (1:1:3) | [88] |
| Averrhoa bilimbi | Agitation | Pectin | ChCl:CA (1:1) | [89] |
| Crude palm oil | LLE | Tocols | ChCl:MalA | [90] |
| Cod skins | Heating and stirring | Collagen peptides | ChCl:U; ChCl:EG; ChCl:Gly; ChCl:LA; ChCl:AA; ChCl:OA | [91] |
| Analyte | Material | Bio-Based Solvent | Method | Ref. |
|---|---|---|---|---|
| Oil | Yarrowia lipolytica | CPME | Hot reflux | [99] |
| Oil | Pistacia Lentiscus L. | MeTHF | Soxhlet | [100] |
| Oil | Anabaena planctonica | D-limonene | Pressurized liquid extraction | [101] |
| Oil | Jatropha curcas L. | DMC | Maceration | [102] |
| Peroxidase enzyme | Momordica charantia | DMC | Three-phase partitioning | [103] |
| Triterpenoids | Betula pendula Roth. | Ethyl acetate | Reflux | [104] |
| Oil | Hura crepitans | Ethyl acetate | Microwave | [105] |
| Curcuminoids | Curcuma longa L. | Ethyl lactate | Maceration | [106] |
| Caffeine | Camellia sinesis | Ethyl lactate | Pressurized liquid extraction | [107] |
| Fatty acids | Arachis Hypogaea | α-pinene | Soxhlet | [108] |
| Material | Analyte | Solvent | T (°C)/P (MPa) | Ref. |
|---|---|---|---|---|
| Rosmarinus officinalis | EO | SFE-CO2 | 40 °C/10.34 MPa; 50 °C/17.24 MPa; | [122] |
| Origanum majorana | EO | SFE-CO2 | 50 °C/45 MPa | [123] |
| Jojoba seeds | oil | SFE-CO2 | 25–45 °C/67–90 MPa | [125] |
| Carapa guianensis | Fatty acids + phenolic | n-butane | 25 °C, 0.7 MP | [128] |
| Carrot peel | carotenoids | SFE-CO2 -Ethanol | 58,5 °C/30.6 MPa (with 14.3% of ethanol) | [129] |
| Helianthus annuus L. | Fatty acids | n-butane | 40 °C, 0.4 MPa | [129] |
| Perilla frutescens | Lipids | n-propane | 40 °C, 0.8 MPa | [131] |
| Sesamum indicum seeds | oil | SFE-CO2 | 19–25 °C/40–60 MPa | [132] |
| Sesamum indicum seeds | Fatty acids + antioxidants + proteins | n-propane | 60 °C, 12 MPa | [132] |
| Euglena gracilis | Lipids | DME | 20 °C, 0.7 MPa | [133] |
| Botryococcus braunii | Hydrocarbons | DME | 20 °C, 0.7 MPa | [134] |
| Arthrospira platensis | lipids | DME | 20 °C, 0.5 MPa | [135] |
| Citrus leaves | Essential oil | DME | 35 °C, 0.78 MPa | [135] |
| Orange Waste | Terpenoids | LPG | 35 °C, 0.45 MPa | [136] |
| Salvia hispanica L. | Fatty acids + antioxidants | n-propane | 45 °C, 10 MPa | [139] |
| Microalgae | Lipids | DME | 30 °C, 0.7 MPa | [140] |
| Ions | Group | Source | Precursor | Structure | Example of IL | Ref. |
|---|---|---|---|---|---|---|
| Anions | Carboxylic acids | Vegetable oils | Oleic acid |  | [HE2A][C18OO] | [151] |
| Amino acids | Meat, eggs and dairy foods | Glycine |  | [C2mim][Gly] | [152] | |
| Lysine |  | [Ch][Lys] | [153] | |||
| Cations | Natural amine | Soybeans, eggs and peanuts | choline |  | [Ch][Ser] | [154] |
| [Ch]Cl | [155] | |||||
| [Ch][Ala] | [156] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24163007
Chemat F, Abert Vian M, Ravi HK, Khadhraoui B, Hilali S, Perino S, Fabiano Tixier A-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules. 2019; 24(16):3007. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24163007
Chicago/Turabian StyleChemat, Farid, Maryline Abert Vian, Harish Karthikeyan Ravi, Boutheina Khadhraoui, Soukaina Hilali, Sandrine Perino, and Anne-Sylvie Fabiano Tixier. 2019. "Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects" Molecules 24, no. 16: 3007. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24163007