Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds
Abstract
:1. Introduction
2. Results
2.1. Triterpene Seco-Acids Decreased Viability of Cancer Cells
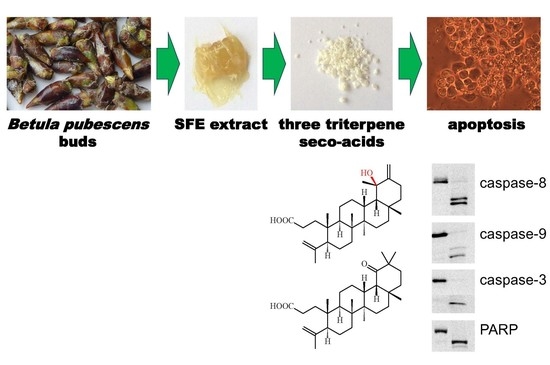
2.2. Triterpene Seco-Acids Increased Apoptosis in AGS and DLD-1 Cells
2.3. Triterpene Seco-Acids Activated Extrinsic and Intrinsic Pathway of Apoptosis in AGS and DLD-1 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Plant Material
4.3. Bud Extraction and Chemical Analysis
4.4. Cell Culture and Treatment
4.5. Cell Viability Assay
4.6. Apoptosis Assay
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Culpeper, N. Culpeper’s Complete Herbal: A Book of Natural Remedies of Ancient Ills; (The Wordsworth Collection Reference Library); NTC/Contemporary Publishing Company (Reprint of 1653 Work): London, UK, 1995. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [PubMed]
- Raal, A. Birch (Betula spp.). In Recent Progress in Medical Plants; Awaad, A.S., Singh., V.K., Govil, J.N., Eds.; Drug Plants II. Studium Press LLC: Houston, TX, USA, 2010; Volume 28, pp. 123–142. [Google Scholar]
- Mashentseva, A.A.; Dehaen, W.; Seitembetov, T.S.; Seitembetova, A.J. Comparison of the antioxidant activity of the different Betula pendula Roth. extracts from northern Kazakhstan. J. Phytol. 2011, 3, 18–25. [Google Scholar]
- Duric, K.; Kovac-Besovic, E.; Niksic, H.; Sofic, E. Antibacterial activity of methanolic extracts, decoction and isolated triterpene products from different parts birch, Betula pendula Roth. J. Plant. Stud. 2013, 2, 61–70. [Google Scholar] [CrossRef]
- Tolmacheva, A.A.; Rogozhin, E.A.; Deryabin, D.G. Antibacterial and quorum sensing regulatory activities of some traditional Eastern-European medicinal plants. Acta Pharm. 2014, 64, 173–186. [Google Scholar] [CrossRef] [Green Version]
- State Pharmacopoeia of USSR, 11-th ed.; Medizina: Moscow, Russia, 1990; Volume 2. (In Russian)
- Goun, E.A.; Petrichenko, V.M.; Solodnikov, S.U.; Suhinina, T.V.; Kline, M.A.; Cunningham, G.; Nguyen, C.; Miles, H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002, 81, 337–342. [Google Scholar] [CrossRef]
- Spiridonov, N.A.; Konovalov, D.A.; Arkhipov, V.V. Cytotoxicity of some Russian ethnomedicinal plants and plant compounds. Phytother. Res. 2005, 19, 428–432. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Moskwa, J.; Borawska, M.H. Anticancer activity of some plant extracts on U87MG human gliobastoma cell. In Book of Abstract of 6th Rus.-Korean Conf. Current Issues of Biologically Active Compound Chemistry and Biotechnology; Novosibirsk Institute of Organic Chemistry: Novosibirsk, Russua, 2015; p. 189. [Google Scholar]
- Isidorov, V.; Szoka, Ł.; Nazaruk, J. Cytotoxicity of white birch bud extracts: Perspectives for therapy of tumors. PLoS ONE 2018, 13, e0201949. [Google Scholar] [CrossRef]
- Vedernikov, D.N.; Roshchin, V.I. Extractive compounds of Betulaceae family buds (Betula pendula Roth.): V. Composition of triterpene seco-acids. Russ. J. Bioorg. Chem. 2012, 38, 762–768. [Google Scholar] [CrossRef]
- Isidorov, V.; Szczepaniak, L.; Wróblewska, A.; Pirożnikow, E.; Vetchinnikova, L. Gas chromatographic-mass spectrometric examination of chemical composition of two Eurasian birch (Betula L.) bud exudates and its taxonomical implication. Biochem. System. Ecol. 2014, 52, 41–48. [Google Scholar] [CrossRef]
- Prokofʹeva, N.G.; Chaikina, E.L.; Pokhilo, N.D.; Anisimov, M.M. Hemolytic and cytotoxic activity of dammarane-type triterpenoids. Chem. Nat. Comp. 2007, 43, 72–75. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Sekiya, M.; Yamazaki, K.; Ikeshiro, Y.; Fujioka, T.; Yamagishi, T.; Kitagawa, S.; Takaishi, Y. Triterpenoids from the floral spikes of Betula platyphylla var. japonica and their reversing activity against multidrug-resistant cancer cells. J. Nat. Prod. 2007, 70, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N. Betulinic acid: Recent advances in chemical modifications, effective delivery, and molecular mechanisms of a promising anticancer therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef]
- Szoka, L.; Karna, E.; Hlebowicz-Sarat, K.; Karaszewski, J.; Boryczka, S.; Palka, J.A. Acetylenic derivative of betulin induces apoptosis in endometrial adenocarcinoma cell line. Biomed. Pharmacother. 2017, 95, 429–436. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Xiang, H.M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant. Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.W.; Kim, Y.C.; Sung, S.H. Cytotoxic activities of naturally occurring oleanane-, ursane-, and lupane-type triterpenes on HepG2 and AGS cells. Pharmacogn. Mag. 2017, 13, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.W.; Ruefl, A.A.; Lowe, S. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011, 18, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Arya, R.K.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.K.; Rana, T.S.; Datta, D. Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. PLoS ONE 2016, 11, e0159430. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.; Stocki, M.; Nazaruk, J.; Bakier, S. Metabolomic GC-MS study of Betula pubescens bud extracts. Trees 2019. submitted. [Google Scholar]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
Sample Availability: Samples of the compounds 1,3 are available from the authors. |




| Compound 2 48 h | Compound 2 72 h | Compound 3 48 h | Compound 3 72 h | |
|---|---|---|---|---|
| Fibroblasts | 128 ± 5.9 | 84 ± 4.7 | 129 ± 6.8 | 79 ± 3.8 |
| A.375 | 101 ± 4.8 | 76 ± 4.4 | 111 ± 6.0 | 53 ± 3.5 |
| Ags | 109 ± 5.4 | 69 ± 3.8 | 113 ± 5.1 | 36 ± 4.2 |
| DLD-1 | 53 ± 4.9 | 29 ± 3.2 | 37 ± 4.1 | 26 ± 2.7 |
| HeLa | 91 ± 5.1 | 33 ± 4.0 | 118 ± 6.1 | 25 ± 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szoka, Ł.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules 2019, 24, 4060. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224060
Szoka Ł, Isidorov V, Nazaruk J, Stocki M, Siergiejczyk L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules. 2019; 24(22):4060. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224060
Chicago/Turabian StyleSzoka, Łukasz, Valery Isidorov, Jolanta Nazaruk, Marcin Stocki, and Leszek Siergiejczyk. 2019. "Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds" Molecules 24, no. 22: 4060. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224060