Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds
Abstract
:1. Introduction
2. Results
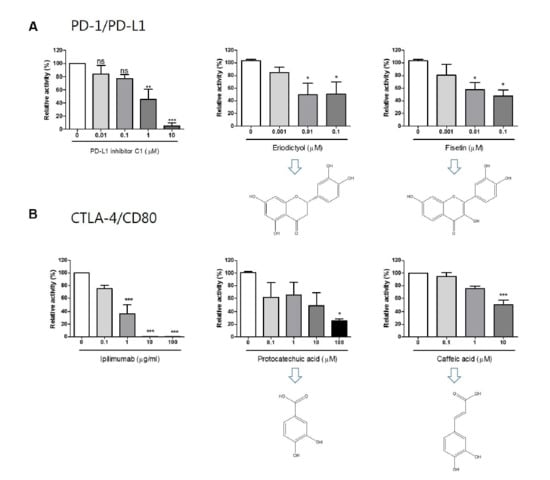
2.1. RVS Blocks the PD-1/PD-L1 Interaction
2.2. RVS Blocks the CTLA-4/CD80 Interaction
2.3. Structural Elucidation of Compounds 1–20
2.4. PD-1/PD-L1 Blocking Effect of Isolated Compounds
2.5. CTLA-4/CD80 Blocking Effect of Isolated Compounds
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation of Chemicals
4.4. Chemicals and Antibodies
4.5. Competitive ELISA
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Niimura, N. Determination of the type of lacquer on East Asian lacquer ware. Int. J. Mass Spectrom. 2009, 284, 93–97. [Google Scholar] [CrossRef]
- Lee, J.D.; Huh, J.E.; Jeon, G.S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 2068–2276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kwon, Y.S.; Chun, W.J.; Kim, T.Y.; Sun, J.; Yu, C.Y.; Kim, M.J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010, 120, 539–543. [Google Scholar] [CrossRef]
- Thompson, C.B.; Allison, J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity 1997, 7, 445–450. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dholaria, B.; Hammond, W.; Shreders, A.; Lou, Y. Emerging therapeutic agents for lung cancer. J. Hematol. Oncol. 2016, 9, 138. [Google Scholar] [CrossRef]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Musielak, B.; Kocik, J.; Skalniak, L.; Magiera-Mularz, K.; Sala, D.; Czub, M.; Stec, M.; Siedlar, M.; Holak, T.A.; Plewka, J. CA-170—A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules 2019, 24, 2804. [Google Scholar] [CrossRef]
- Skalniak, L.; Zak, K.M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M.; et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget 2017, 8, 72167–72181. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Choi, S.U.; Pang, C.; Kim, S.Y.; Lee, K.R. Identification of cytotoxic and anti-inflammatory constituents from the bark of Toxicodendron vernicifluum (Stokes) F.A. Barkley. J. Ethnopharmacol. 2015, 162, 231–237. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, I.S.; Kim, S.A.; Na, C.S.; Hong, C.Y.; Dong, M.S.; Yoo, H.H. Inhibitory effect of Rhus verniciflua Stokes extract on human aromatase activity; butin is its major bioactive component. Bioorg. Med. Chem. Lett. 2014, 24, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Choi, J.H.; Yang, H.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Park, J.G.; Kim, S.; Kweon, H.Y.; Seo, S.; Na, D.S.; Lee, D.; Hong, C.Y.; Na, C.S.; Dong, M.S.; et al. Extract of Rhus verniciflua stokes protects the diet-induced hyperlipidemia in mice. Arch. Pharm. Res. 2015, 38, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Wilde, A.; Ebmeyer, J.; Skouroumounis, G.K.; Taylor, D. Examination of the phenolic profile and antioxidant activity of the leaves of the Australian native plant Smilax glyciphylla. J. Nat. Prod. 2013, 76, 1930–1936. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, C.W.; Kim, J.H.; Lee, J.C.; An, W.G. Extract of Rhus verniciflua Stokes Induces p53-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Evid. Based Complement Alternat Med. 2019, 9407340. [Google Scholar] [CrossRef]
- Kang, Y.; Yoon, S.W.; Park, B. Allergen-removed Rhus verniciflua Stokes suppresses invasion and migration of pancreatic cancer cells through downregulation of the JAK/STAT and Src/FAK signaling pathways. Oncol. Rep. 2018, 40, 3060–3068. [Google Scholar] [CrossRef]
- Chae, J.; Lee, S.; Lee, S. Potential Efficacy of Allergen Removed Rhus Verniciflua Stokes Extract to Maintain Progression-Free Survival of Patients With Advanced Hepatobiliary Cancer. Explore 2018, 14, 300–304. [Google Scholar] [CrossRef]
- Lee, K.W.; Um, E.S.; Jung, B.B.; Choi, E.S.; Kim, E.Y.; Lee, S.; Jang, E.; Lee, J.H.; Kim, Y. Rhus verniciflua Stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol. Rep. 2018, 39, 1141–1147. [Google Scholar] [CrossRef]
- Kang, S.H.; Hwang, I.H.; Son, E.; Cho, C.K.; Choi, J.S.; Park, S.J.; Jang, B.C.; Lee, K.B.; Lee, Z.W.; Lee, J.H.; et al. Allergen-removed Rhus verniciflua extract induces ovarian cancer cell death via JNK activation. Am. J. Chin. Med. 2016, 44, 1719–1735. [Google Scholar] [CrossRef]
- Jang, I.S.; Park, J.W.; Jo, E.B.; Cho, C.K.; Lee, Y.W.; Yoo, H.S.; Park, J.; Kim, J.; Jang, B.C.; Choi, J.S. Growth inhibitory and apoptosis-inducing effects of allergen-free Rhus verniciflua Stokes extract on A549 human lung cancer cells. Oncol. Rep. 2016, 36, 3037–3043. [Google Scholar] [CrossRef]
- Suruga, T.; Hiruma, W.; Kadokura, K.; Tomita, T.; Miyata, A.; Sekino, Y.; Kimura, M.; Yamaguchi, N.; Murai, T.; Komatsu, Y.; et al. Antitumor and apoptopic effects of a plant exact mixture containing Rhus verniciflua and other herbs in human leukemia cells. Drug Res. 2017, 67, 127–130. [Google Scholar]
- Lee, S.K.; Jung, H.S.; Eo, W.K.; Lee, S.Y.; Kim, S.H.; Shim, B.S. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: Report of two cases. Ann. Oncol. 2010, 21, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Moon, J.W.; Lee, S.K.; Kim, S.M.; Kim, N.; Ko, S.G.; Kim, H.S.; Park, S.H. Rhus verniciflua extract modulates survival of MCF-7 breast cancer cells through the modulation of AMPK-pathway. Biol. Pharm. Bull. 2014, 37, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Chung, K.S.; Seo, J.H.; Yim, S.V.; Park, H.J.; Choi, J.H.; Lee, K.T. Sulfuretin from heartwood of Rhus verniciflua triggers apoptosis through activation of Fas, Caspase-8, and the mitochondrial death pathway in HL-60 human leukemia cells. J. Cell. Biochem. 2012, 113, 2835–2844. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, C.H.; Jang, B.H.; Go, H.Y.; Park, J.H.; Choi, Y.K.; Hong, S.; Shin, Y.C.; Ko, S.G. Selective cytotoxic effects on human cancer cell lines of phenolic-rich ethyl-acetate fraction from Rhus verniciflua Stokes. Am. J. Chin. Med. 2009, 37, 609–620. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.P.; Jung, C.H.; Hong, M.H.; Hong, M.C.; Bae, H.S.; Lee, S.D.; Park, S.Y.; Park, J.H.; Ko, S.G. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua Stokes in AGS gastric cancer cells. Int. J. Mol. Med. 2006, 18, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Jesus, A.R.; Vila-Viçosa, D.; Machuqueiro, M.; Marques, A.P.; Dore, T.M.; Rauter, A.P. Targeting Type 2 Diabetes with C-Glucosyl Dihydrochalcones as Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: Synthesis and Biological Evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef]
- Swart, M.; Verbrugge, I.; Beltman, J.B. Combination Approaches with Immune-Checkpoint Blockade in Cancer Therapy. Front Oncol. 2016, 6, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Xu, J.; Wang, Z.; Gong, Z.; Liu, J.; Zheng, Y.; Li, J.; Li, J. Epitope mapping reveals the binding mechanism of a functional antibody cross-reactive to both human and murine programmed death 1. MAbs 2017, 9, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.Y.; Tanaka, Y.; Iwasaki, M.; Gittis, A.G.; Su, H.P.; Mikami, B.; Okazaki, T.; Honjo, T.; Minato, N.; Garboczi, D.N. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 3011–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bajorath, J.; Flies, D.B.; Dong, H.; Honjo, T.; Chen, L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 2003, 197, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef]
- Hurwitz, A.A.; Foster, B.A.; Kwon, E.D.; Truong, T.; Choi, E.M.; Greenberg, N.M.; Burg, M.B.; Allison, J.P. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000, 60, 2444–2448. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Kim, T.I.; Kim, J.H.; Chung, H.-S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019, 24, 4062. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224062
Li W, Kim TI, Kim JH, Chung H-S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules. 2019; 24(22):4062. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224062
Chicago/Turabian StyleLi, Wei, Tae In Kim, Ji Hye Kim, and Hwan-Suck Chung. 2019. "Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds" Molecules 24, no. 22: 4062. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224062