Graphene Oxide@3D Hierarchical SnO2 Nanofiber/Nanosheets Nanocomposites for Highly Sensitive and Low-Temperature Formaldehyde Detection
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of Sensing Materials
2.2. Gas Sensing Properties
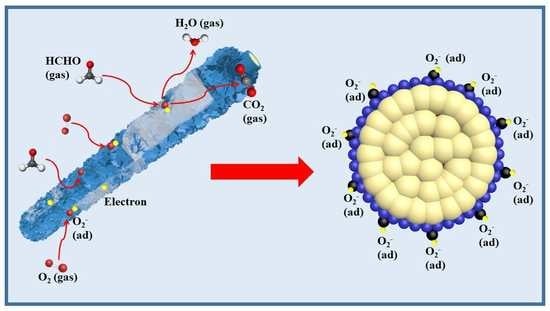
2.3. Gas Sensing Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sensing Materials
3.3. Characterization
3.4. Gas Sensor Fabrication and Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators B 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, H.; Chang, Y.; Zhao, Y.; Li, M.; Wang, C.; Sun, Y.; Liu, J. One-step synthesis of Au/SnO2/RGO nanocomposites and their VOC sensing properties. IEEE Trans. Nanotechnol. 2018, 17, 212–219. [Google Scholar] [CrossRef]
- Yoosefian, M.; Raissi, H.; Mola, A. The hybrid of Pd and SWCNT (Pd loaded on SWCNT) as an efficient sensor for the formaldehyde molecule detection: A DFT study. Sens. Actuators B 2015, 212, 55–62. [Google Scholar] [CrossRef]
- Chung, P.R.; Tzeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde gas sensors: A review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [Green Version]
- Rong, X.; Chen, D.; Qu, G.; Li, T.; Zhang, R.; Sun, J. Effects of graphene on the microstructures of SnO2@rGO nanocomposites and their formaldehyde-sensing performance. Sens. Actuators B 2018, 269, 223–237. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Chen, Z.; Li, H.; Chen, A.; Wang, X.; Yang, J. Enhanced formaldehyde sensing properties of hollow SnO2 nanofibers by graphene oxide. Sens. Actuators B 2017, 250, 533–542. [Google Scholar] [CrossRef]
- Wan, K.C.; Wang, D.; Wang, F.; Li, H.J.; Xu, J.C.; Wang, X.Y.; Yang, J.H. Hierarchical In2O3@SnO2 core-shell nanofiber for high efficiency formaldehyde detection. ACS Appl. Mater. Int. 2019, 11, 45214–45225. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Wang, R.; Zhang, T. A formaldehyde sensor: Significant role of pn heterojunction in gas-sensitive core-shell nanofibers. Sens. Actuators B 2018, 258, 1230–1241. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Zou, X.; Li, G.-D. Tungsten oxide clusters decorated ultrathin In2O3 nanosheets for selective detecting formaldehyde. Sens. Actuators B 2017, 252, 232–238. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Xie, T.; Yuan, Z.; Liu, C.; Jiang, Y. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B 2016, 223, 149–156. [Google Scholar] [CrossRef]
- Upadhyay, S.; Mishra, R.; Sahay, P. Cr-doped WO3 nanosheets: Structural, optical and formaldehyde sensing properties. Ceram. Int. 2016, 42, 15301–15310. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cui, X.; Wang, Y.; Gao, Y.; Sun, P.; Liu, F.; Shimanoe, K.; Yamazoe, N.; Lu, G. Enhanced gas sensing properties to acetone vapor achieved by α-Fe2O3 particles ameliorated with reduced graphene oxide sheets. Sens. Actuators B 2017, 241, 904–914. [Google Scholar] [CrossRef]
- Zhao, L.K.; Wang, K.; Wei, W.; Wang, L.L.; Han, W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat 2019, 1, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Gaikwad, G.; Patil, P.; Patil, D.; Naik, J. Synthesis and evaluation of gas sensing properties of PANI based graphene oxide nanocomposites. Mater. Sci. Eng. B 2017, 218, 14–22. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, Y.; Ding, J.; Zhang, J.; Zhang, M.; Zhu, Y.; Li, H. Zinc oxide nanoparticle incorporated graphene oxide as sensing coating for interferometric optical microfiber for ammonia gas detection. Sens. Actuators B 2018, 254, 239–247. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J.; Gaskov, A.M. Reduced graphene oxide (rGO) encapsulated Co3O4 composite nanofibers for highly selective ammonia sensors. Sens. Actuators B 2016, 222, 864–870. [Google Scholar] [CrossRef]
- Guo, L.; Kou, X.; Ding, M.; Wang, C.; Dong, L.; Zhang, H.; Feng, C.; Sun, Y.; Gao, Y.; Sun, P. Reduced graphene oxide/α-Fe2O3 composite nanofibers for application in gas sensors. Sens. Actuators B 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.-L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X. Magnetically separable ZnFe2O4–graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind. Eng. Chem. Res. 2011, 50, 7210–7218. [Google Scholar] [CrossRef]
- Liu, F.; Chu, X.; Dong, Y.; Zhang, W.; Sun, W.; Shen, L. Acetone gas sensors based on graphene-ZnFe2O4 composite prepared by solvothermal method. Sens. Actuators B 2013, 188, 469–474. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Zong, X.; Dong, G.; Zhang, Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sens. Actuators B 2018, 262, 531–541. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumar, A.; Thakur, A.D. Free standing graphene oxide films for gas sensing applications. Mater. Today Proc. 2018, 5, 732–736. [Google Scholar] [CrossRef]
- Jung, M.W.; Kang, S.M.; Nam, K.-H.; An, K.-S.; Ku, B.-C. Highly transparent and flexible NO2 gas sensor film based on MoS2/rGO composites using soft lithographic patterning. Appl. Surf. Sci. 2018, 456, 7–12. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Guo, F.; Liu, S.; Qi, L.; Shan, M.; Fan, X. Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Ye, Z.; Liu, C.; Xie, G.; Du, X.; Jiang, Y. Novel highly sensitive QCM humidity sensor with low hysteresis based on graphene oxide (GO)/poly (ethyleneimine) layered film. Sens. Actuators B 2016, 234, 145–154. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Cui, Y.; Zhu, J.-H.; Han, B.-H. Preparation of three-dimensional graphene oxide–polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Int. 2013, 5, 9172–9179. [Google Scholar] [CrossRef]
- Huang, H.; Lü, S.; Zhang, X.; Shao, Z. Glucono-δ-lactone controlled assembly of graphene oxide hydrogels with selectively reversible gel–sol transition. Soft Matter 2012, 8, 4609–4615. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Q.-Y.; Han, B.-H. Solvothermal synthesis of homogeneous graphene dispersion with high concentration. Carbon 2011, 49, 3920–3927. [Google Scholar] [CrossRef]
- Zhao, C.; Gong, H.; Lan, W.; Ramachandran, R.; Xu, H.; Liu, S.; Wang, F. Facile synthesis of SnO2 hierarchical porous nanosheets from graphene oxide sacrificial scaffolds for high-performance gas sensors. Sens. Actuators B 2018, 258, 492–500. [Google Scholar] [CrossRef]
- Unwin, P.R.; Güell, A.G.; Zhang, G. Nanoscale electrochemistry of sp2 carbon materials: From graphite and graphene to carbon nanotubes. Acc. Chem. Res. 2016, 49, 2041–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Li, Y.; Wang, J.; Ouyang, Z.; Li, J.; Wei, G.; Su, Z. Interactive oxidation–reduction reaction for the in situ synthesis of graphene–phenol formaldehyde composites with enhanced properties. ACS Appl. Mater. Int. 2014, 6, 4254–4263. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bai, S.; Tian, Y.; Zhao, Y.; Han, N.; Luo, R.; Li, D.; Chen, A. Hybridization of ZnSnO3 and rGO for improvement of formaldehyde sensing properties. Sens. Actuators B 2018, 257, 29–36. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Wu, J. Layer-by-layer assembled In2O3 nanocubes/flower-like MoS2 nanofilm for room temperature formaldehyde sensing. Sens. Actuators B 2018, 273, 176–184. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, P.; Liu, T.; Feng, Y.; Blackman, C.; Li, D. Facile synthesis of mesoporous hierarchical Co3O4–TiO2 p–n heterojunctions with greatly enhanced gas sensing performance. J. Mater. Chem. A 2017, 5, 10387–10397. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, Y.; Guan, X.X.; Cao, J.X.; Li, W.L.; Long, X.; Tan, X. Enhanced performances of PbS quantum-dots-modified MoS2 composite for NO2 detection at room temperature. ACS Appl. Mater. Int. 2017, 11, 438–9447. [Google Scholar]
- Levêque, G.L.; Abanades, S.P.; Jumas, J.-C.; Olivier-Fourcade, J. Characterization of two-step tin-based redox system for thermochemical fuel production from solar-driven CO2 and H2O splitting cycle. Ind. Eng. Chem. Res 2014, 53, 5668–5677. [Google Scholar] [CrossRef]
- Kim, H.W.; Na, H.G.; Kwon, Y.J.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Wu, P.; Kim, S.S. Microwave-assisted synthesis of graphene–SnO2 nanocomposites and their applications in gas sensors. ACS Appl. Mater. Int. 2017, 9, 31667–31682. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jang, B.-H.; Lee, S.-J.; Min, B.K.; Rothschild, A.; Kim, I.-D. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Int. 2014, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.; Xiang, L.; Xie, D. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted and wearable NO2 e-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.-J.; Ziegler, J.R.; Jaegermann, W. Nanostructured SnO2–ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Fomekong, R.L.; Kamta, H.T.; Lambi, J.N.; Lahem, D.; Eloy, P.; Debliquy, M.; Delcorte, A. A sub-ppm level formaldehyde gas sensor based on Zn-doped NiO prepared by a co-precipitation route. J. Alloys Compd. 2018, 731, 1188–1196. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, W.; Wang, L.; Ryu, S.O.; Gu, C. Preparation of porous flower-like SnO2 micro/nano structures and their enhanced gas sensing property. J. Alloys Compd. 2015, 653, 611–618. [Google Scholar] [CrossRef]
- Han, M.; Liu, W.; Qu, Y.; Du, L.; Wei, H. Graphene oxide–SnO2 nanocomposite: Synthesis, characterization, and enhanced gas sensing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 16973–16980. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Zou, X.; Bai, N.; Cao, Y.; Cao, Y.; Fan, M.; Li, G.-D. Platinum dioxide activated porous SnO2 microspheres for the detection of trace formaldehyde at low operating temperature. Sens. Actuators B 2017, 244, 475–481. [Google Scholar] [CrossRef]
- Chen, S.; Qiao, Y.; Huang, J.; Yao, H.; Zhang, Y.; Li, Y.; Du, J.; Fan, W. One-pot synthesis of mesoporous spherical SnO2@ graphene for high-sensitivity formaldehyde gas sensors. RSC Adv. 2016, 6, 25198–25202. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Hong, Y.-Y.; Lin, Z.-D.; Liu, L.-M.; Zhang, X.-W. Enhanced formaldehyde gas sensing properties of ZnO nanosheets modified with graphene. Electron. Mater. Lett. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Du, H.; Yao, P.; Sun, Y.; Wang, J.; Wang, H.; Yu, N. Electrospinning hetero-nanofibers In2O3/SnO2 of homotype heterojunction with high gas sensing activity. Sensors 2017, 17, 1822. [Google Scholar] [CrossRef]
- Mishra, R.K.; Murali, G.; Kim, T.-H.; Kim, J.H.; Lim, Y.J.; Kim, B.-S.; Sahay, P.; Lee, S.H. Nanocube In2O3@ RGO heterostructure based gas sensor for acetone and formaldehyde detection. RSC Adv. 2017, 7, 38714–38724. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Tian, L.; Li, H.; Wan, K.; Yu, X.; Wang, P.; Chen, A.; Wang, X.; Yang, J. Mesoporous ultrathin SnO2 nanosheets in situ modified by graphene oxide for extraordinary formaldehyde detection at low temperatures. ACS Appl. Mater. Int. 2019, 11, 12808–12818. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fan, H.; Tian, H.; Fang, J.; Qian, X. The n-ZnO/n-In2O3 heterojunction formed by a surface-modification and their potential barrier-control in methanal gas sensing. Sens. Actuators B 2016, 222, 508–516. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, X.; Zhang, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. High selectivity to ppb-level HCHO sensor based on mesoporous tubular SnO2 at low temperature. Sens. Actuators B 2017, 247, 664–672. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sens. Actuators B 2017, 244, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-J.; Fuchs, F.; Demadrille, R.; Grévin, B.; Jang, B.-H.; Lee, S.-J.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Fast responding exhaled-breath sensors using WO3 hemitubes functionalized by graphene-based electronic sensitizers for diagnosis of diseases. ACS Appl. Mater. Int. 2014, 6, 9061–9070. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Sensing Materials | Sn 3d | Binding Energy (eV) | Oxygen Species | Relative Percentage (%) | Binding Energy (eV) |
|---|---|---|---|---|---|
| SnO2 NF/NSs | Sn4+ 3d3/2 | 494.7 | Olat | 69.76 | 530.2 |
| Sn4+ 3d5/2 | 486.3 | Oads | 30.24 | 531.3 | |
| 1% GO@SnO2 NF/NSs | Sn4+ 3d3/2 | 494.9 | Olat | 49.14 | 530.3 |
| Sn4+ 3d5/2 | 494.5 | Oads | 50.86 | 531.3 |
| Sensing Materials | T (°C) | LOD (ppm) | Concentration (ppm) | Response (Ra/Rg) | References |
|---|---|---|---|---|---|
| GO/SnO2 | 120 | 0.25 | 100 | 32 | [6] |
| Flower-like SnO2 | 240 | 1 | 100 | 24.8 | [45] |
| GO-SnO2 | 150 | 10 | 200 | 90 | [46] |
| Porous PtO2/SnO2 | 100 | 0.1 | 100 | 70 | [47] |
| SnO2@graphene | 120 | 1 | 100 | 45 | [48] |
| Graphene/ZnO | 200 | 2 | 100 | 12 | [49] |
| In2O3/SnO2 | 275 | 0.5 | 10 | 8.7 | [50] |
| In2O3@rGO | 225 | 5 | 100 | 1.8 | [51] |
| VG/SnO2 | 160 | 0.02 | 100 | 138 | [5] |
| GO@SnO2 NF/NSs | 60 | 0.25 | 100 | 280 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, K.; Yang, J.; Wang, D.; Wang, X. Graphene Oxide@3D Hierarchical SnO2 Nanofiber/Nanosheets Nanocomposites for Highly Sensitive and Low-Temperature Formaldehyde Detection. Molecules 2020, 25, 35. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010035
Wan K, Yang J, Wang D, Wang X. Graphene Oxide@3D Hierarchical SnO2 Nanofiber/Nanosheets Nanocomposites for Highly Sensitive and Low-Temperature Formaldehyde Detection. Molecules. 2020; 25(1):35. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010035
Chicago/Turabian StyleWan, Kechuang, Jialin Yang, Ding Wang, and Xianying Wang. 2020. "Graphene Oxide@3D Hierarchical SnO2 Nanofiber/Nanosheets Nanocomposites for Highly Sensitive and Low-Temperature Formaldehyde Detection" Molecules 25, no. 1: 35. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010035