Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review
Abstract
:1. Introduction
2. Application of Curcumin for Cancer Therapy
3. Challenges Associated with Curcumin Delivery
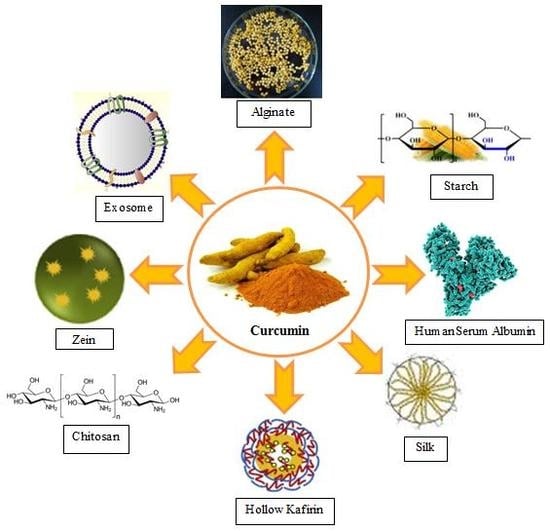
4. Biopolymer Nanoparticles (NPs)
4.1. Protein-Based Biopolymers
4.1.1. Albumin
4.1.2. Zein-Based NP
4.1.3. Silk-Based NPs
4.1.4. Other Protein-Based NPs
4.2. Polysaccharide NPs
4.2.1. Chitosan
4.2.2. Alginate
4.2.3. Starch
4.2.4. Cellulose
5. Exosomes
5.1. Advantage and Disadvantage of Exosomes
5.2. Exosomes for Curcumin Delivery
6. Co-Polymers
7. Targeted Delivery
8. Conclusions and Future Trends
Funding
Conflicts of Interest
Abbreviations
| VEGF | Vascular endothelial growth factor |
| HAS | human serum albumin |
| BSA | bovine serum albumin |
| NPs | nanoparticles |
| HER2 | human epidermal growth factor receptor 2 |
| HA | hyaluronic acid |
| Apt-HSA/CCM | aptamer-decorated curcumin-loaded human serum albumin |
| SSPS | soluble soybean polysaccharide |
| Cur-ACRU/CS | curcumin-loaded acylated cruciferin/charged chitosan |
| CDG-CANPs | curcumin diethyl diglutarate-loaded Chitosan/alginate NPs |
| CUR-AlgNP | curcumin loaded alginate NP |
| Cur-CS/Alg NPs | curcumin-loaded chitosan/alginate NPs |
| CMC | carboxymethyl cellulose |
| Cur-NLCs | curcumin loaded nanostructured lipid carriers |
| ANC | aminated nanocellulose |
| EWP | egg white protein |
| PECs | polyelectrolyte complexes |
| SC | sodium caseinate |
| SA | sodium alginate |
| PEG | poly (ethylene glycol) |
| PCL | poly (e-caprolactone) |
| FDA | U.S. Food and Drug administration |
References
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Ahmadi, Z.; Tavakol, S.; Ashrafizadeh, M. Berberine as a potential autophagy modulator. J. Cell. Physiol. 2019, 234, 1491414926. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 18451865. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, Z.; Mohammadinejad, R.; Ashrafizadeh, M. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J. Drug Delivery Sci. Technol. 2019, 51, 591–604. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728. [Google Scholar] [CrossRef]
- Murray-Stewart, T.; Casero, R. Regulation of polyamine metabolism by curcumin for cancer prevention and therapy. Med. Sci. 2017, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Montalbán, M.; Coburn, J.; Lozano-Pérez, A.; Cenis, J.; Víllora, G.; Kaplan, D. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- De Matos, R.P.A.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of curcumin-nanoemulsion associated with photodynamic therapy in cervical carcinoma cell lines. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramayanti, O.; Brinkkemper, M.; Verkuijlen, S.; Ritmaleni, L.; Go, M.; Middeldorp, J. Curcuminoids as EBV lytic activators for adjuvant treatment in EBV-positive carcinomas. Cancers 2018, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Tan, B.K.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. In Seminars in Cancer Biology; Theresa, V., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 40, pp. 48–81. [Google Scholar]
- Oskouie, M.N.; Aghili Moghaddam, N.S.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J. Cell. Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef]
- Hu, X.; Huang, F.; Szymusiak, M.; Liu, Y.; Wang, Z.J. Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity. J. Pharmacol. Exp. Ther. 2015, 352, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an anti-cancer agent: Review of the gap between basic and clinical applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahed, V.; Zarrabi, A.; Bordbar, A.-K.; Hafezi, M.S. NMR (1H, ROESY) spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin and curcumin. Food Chem. 2014, 165, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, H.; Movahedi, B.; Zarrabi, A.; Jahandar, M. A multifunctional hierarchically assembled magnetic nanostructure towards cancer nano-theranostics. RSC Adv. 2015, 5, 77255–77263. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef]
- Tavakol, S.; Zare, S.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M. The impact of the particle size of curcumin nanocarriers and the ethanol on beta_1-integrin overexpression in fibroblasts: A regenerative pharmaceutical approach in skin repair and anti-aging formulations. DARU J. Pharm. Sci. 2019, 27, 159–168. [Google Scholar] [CrossRef]
- Ajdary, M.; Moosavi, M.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.J.N. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J.J.A. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [Green Version]
- Tavakol, S.; Kiani, V.; Tavakol, B.; Derakhshan, M.A.; Joghataei, M.T.; Rezayat, S.M. Toxicity Concerns of Nanocarriers. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Vijay, M., Prashant, K., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 453–484. [Google Scholar]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Nadimi, A.E.; Ebrahimipour, S.Y.; Afshar, E.G.; Falahati-Pour, S.K.; Ahmadi, Z.; Mohammadinejad, R.; Mohamadi, M. Nano-scale drug delivery systems for antiarrhythmic agents. Eur. J. Med. Chem. 2018, 157, 1153–1163. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Kotla, N.G.; Afshar, E.G.; Samarghandian, S.; Mandegary, A.; Pardakhty, A.; Mohammadinejad, R.; Sethi, G. Nanoparticles targeting STATs in cancer therapy. Cells 2019, 8, 1158. [Google Scholar] [CrossRef] [Green Version]
- Tavakol, S.; Ashrafizadeh, M.; Deng, S.; Azarian, M.; Abdoli, A.; Motavaf, M.; Poormoghadam, D.; Khanbabaei, H.; Ghasemipour Afshar, E.; Mandegary, A. Autophagy modulators: Mechanistic aspects and drug delivery systems. Biomolecules 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mat. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nut. 2018, 59, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef]
- Tavakol, S. Acidic pH derived from cancer cells may induce failed reprogramming of normal differentiated cells adjacent tumor cells and turn them into cancer cells. Med. Hypotheses 2014, 83, 668–672. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, C.; Xiong, H.; Du, S.; Zhou, J.; Yin, L.; Yao, J. Dose-reduction antiangiogenic curcumin-low molecular weight heparin nanodrugs for enhanced combinational antitumor therapy. Eur. J. Pharm. Sci. 2018, 119, 121–134. [Google Scholar] [CrossRef]
- Rabiee, S.; Tavakol, S.; Barati, M.; Joghataei, M.T. Autophagic, apoptotic, and necrotic cancer cell fates triggered by acidic pH microenvironment. J. Cell. Physiol. 2019, 234, 12061–12069. [Google Scholar] [CrossRef]
- Seca, A.; Pinto, D. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Alemi, A.; Reza, J.Z.; Haghiralsadat, F.; Jaliani, H.Z.; Karamallah, M.H.; Hosseini, S.A.; Karamallah, S.H. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J. Nanobiotechnol. 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Imanifard, S.; Zarrabi, A.; Zarepour, A.; Jafari, M.; Khosravi, A.; Razmjou, A. Nanoengineered Thermoresponsive Magnetic Nanoparticles for Drug Controlled Release. Macromol. Chem. Phys. 2017, 218, 1700350. [Google Scholar] [CrossRef]
- Cancer Therapy. Available online: https://sarinamedtrip.com/ (accessed on 5 February 2020).
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Kaviyani, N.; Tavakol, S. Monoterpenes modulating autophagy: A review study. Basic Clin. Pharmacol. Toxicol. 2020, 126, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.-L.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of β-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-κB ligand through modulation of NF-κB pathway. Int. J. Cancer 2008, 123, 1733–1740. [Google Scholar] [CrossRef]
- Manna, S.K.; Aggarwal, R.S.; Sethi, G.; Aggarwal, B.B.; Ramesh, G.T. Morin (3,5,7,2′,4′-pentahydroxyflavone) abolishes nuclear factor-κB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-κB–regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. 2007, 13, 2290–2297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. MicroRNAs mediate the anti-tumor and protective effects of ginsenosides. Nutr. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Nano-soldiers Ameliorate Silibinin Delivery: A Review Study. Curr. Drug Deliv. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-κB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-κB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Jahandar, M.; Zarrabi, A.; Shokrgozar, M.A.; Mousavi, H. Synthesis, characterization and application of polyglycerol coated Fe3O4 nanoparticles as a nano-theranostics agent. Mater. Res. Express 2015, 2, 125002. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamamdinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 1–18. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef] [PubMed]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Leśniewska, D.M.; Bałan, B.J.; Skopiński, P. Angiogenesis modulation by exogenous antioxidants. Central-Eur. J. Immunol. 2017, 42, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Gevrek, F.; Erdemir, F. Investigation of the effects of curcumin, vitamin E and their combination in cisplatin-induced testicular apoptosis using immunohistochemical technique. Turkish J. Urol. 2018, 44, 16. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.C.; Mock, C.D.; Thilagavathi, R.; Selvam, C. Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sci. 2016, 152, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.-T.; Liu, H.-F.; Liu, Z.-G.; Xiao, P.; Chen, J.-J.; Tan, Y.; Jiang, X.-X.; Jiang, Z.-C.; Qiu, Y.; Huang, H.-J. Curcumin downregulates the expression of Snail via suppressing Smad2 pathway to inhibit TGF-β1-induced epithelial-mesenchymal transitions in hepatoma cells. Oncotarget 2017, 8, 108498. [Google Scholar] [CrossRef] [Green Version]
- Pastorelli, D.; Fabricio, A.S.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based Biomaterials for tissue engineering and regenerative medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V.; Reddy, B.S. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar] [PubMed]
- Arya, G.; Das, M.; Sahoo, S.K. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 2018, 102, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, A.; Ashrafizadeh, M.; Afshar, E.G.; Pardakhty, A.; Mandegary, A.; Mohammadinejad, R.; Sethi, G. Topoisomerase inhibitors: Pharmacology and emerging nanoscale delivery systems. Pharmacol. Res. 2020, 151, 104551. [Google Scholar] [CrossRef] [PubMed]
- Salehiabar, M.; Nosrati, H.; Javani, E.; Aliakbarzadeh, F.; Manjili, H.K.; Davaran, S.; Danafar, H. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Elias, E.J.; Anil, S.; Ahmad, S.; Daud, A. Colon targeted curcumin delivery using guar gum. Nat. Product Commun. 2010, 5, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Doostmohammadi, M.; Ameri, A.; Mohammadinejad, R.; Dehghannoudeh, N.; Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Hydrogels for peptide hormones delivery: Therapeutic and tissue engineering applications. Drug Des. Dev. Ther. 2019, 13, 3405–3418. [Google Scholar] [CrossRef] [Green Version]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-Encapsulation of Plant Growth-Promoting Rhizobacteria and Their Metabolites Using Alginate-Silica Nanoparticles and Carbon Nanotube Improves UCB1 Pistachio Micropropagation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103. [Google Scholar]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the formulation of alginate-gelatin encapsulated pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.A.; Sohrevardi, S.M.; Mandegary, A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef]
- Zou, L.; Xie, A.; Zhu, Y.; McClements, D.J. Cereal proteins in nanotechnology: Formulation of encapsulation and delivery systems. Curr. Opin. Food Sci. 2019, 25, 28–34. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakol, S.; Rasoulian, B.; Ramezani, F.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M. Core and biological motif of self-assembling peptide nanofiber induce a stronger electrostatic interaction than BMP2 with BMP2 receptor 1A. Mater. Sci. Eng. C 2019, 101, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Saber, R.; Hoveizi, E.; Tavakol, B.; Aligholi, H.; Ai, J.; Rezayat, S.M. Self-assembling peptide nanofiber containing long motif of laminin induces neural differentiation, tubulin polymerization, and neurogenesis: In vitro, ex vivo, and in vivo studies. Mol. Neurobiol. 2016, 53, 5288–5299. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Mousavi, S.M.M.; Tavakol, B.; Hoveizi, E.; Ai, J.; Sorkhabadi, S.M.R. Mechano-transduction signals derived from self-assembling peptide nanofibers containing long motif of laminin influence neurogenesis in in-vitro and in-vivo. Mol. Neurobiol. 2017, 54, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, S.; Nikpour, M.R.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M.; Adabi, M.; Abokheili, S.S.; Jahanshahi, M. Investigating the effects of particle size and chemical structure on cytotoxicity and bacteriostatic potential of nano hydroxyapatite/chitosan/silica and nano hydroxyapatite/chitosan/silver; as antibacterial bone substitutes. J. Nanopart. Res. 2014, 16, 2622. [Google Scholar] [CrossRef]
- Nitta, S.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, B.; Guo, J.; Guo, X.; Sun, X.; Rao, C.; Liu, C.; Zhang, J.; Zhang, C.; Fan, Y.-Y.; Li, W. (NaPO3) 6-assisted formation of dispersive casein-amorphous calcium phosphate nanoparticles: An excellent platform for curcumin delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101412. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A.; Kumar, N.N.; Priyadarsini, K. Preparation of albumin nanoparticles: Optimum size for cellular uptake of entrapped drug (Curcumin). Colloids Surf. A: Physicochem. Eng. Asp. 2019, 567, 86–95. [Google Scholar] [CrossRef]
- Yao, K.; Chen, W.; Song, F.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocolloids 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Huang, G.; Liu, J.; Slavin, M.; Yu, L.L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocolloids 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocolloids 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocolloids 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Zhang, J.; Zheng, Z.; Kaplan, D.L.; Li, G.; Wang, X. Oral delivery of curcumin using silk nano-and microparticles. ACS Biomater. Sci. Eng. 2018, 4, 3885–3894. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-assembly of protein nanoparticles from rice bran waste and their use as delivery system for curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Sun, C.; Dai, L.; Yang, S.; Wei, Y.; Mao, L.; Yuan, F.; Gao, Y. Effect of molecular weight of hyaluronan on zein-based nanoparticles: Fabrication, structural characterization and delivery of curcumin. Carbohydr. Polym. 2018, 201, 599–607. [Google Scholar] [CrossRef]
- Pan, K.; Chen, H.; Baek, S.J.; Zhong, Q. Self-assembled curcumin-soluble soybean polysaccharide nanoparticles: Physicochemical properties and in vitro anti-proliferation activity against cancer cells. Food Chem. 2018, 246, 82–89. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Ju, X.; Udenigwe, C.C.; He, R. Polyelectrolyte complex nanoparticles from chitosan and acylated rapeseed cruciferin protein for curcumin delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Anila, M.; Franklin, S. Synthesis characterization and biological evaluation of alginate nanoparticle for the targeted delivery of curcumin. Mater. Sci. Eng. C 2017, 78, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic effects of photo-irradiation and curcumin-chitosan/alginate nanoparticles on tumor necrosis factor-alpha-induced psoriasis-like proliferation of keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Athira, G.K.; Jyothi, A.N.; Vishnu, V.R. Water Soluble octenyl succinylated cassava starch-curcumin nanoformulation with enhanced bioavailability and anticancer potential. Starch-Stärke 2018, 70, 1700178. [Google Scholar] [CrossRef]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.-N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Kang, N.-W.; Kim, M.-H.; Sohn, S.-Y.; Kim, K.-T.; Park, J.-H.; Lee, S.-Y.; Lee, J.-Y.; Kim, D.-D. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018, 182, 245–258. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdagi, S.I.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Hu, Z.; Cai, J.; Qin, X. Maillard-reacted whey protein isolates and epigallocatechin gallate complex enhance the thermal stability of the pickering emulsion delivery of curcumin. J. Agri. Food Chem. 2019, 67, 5212–5220. [Google Scholar] [CrossRef]
- Tran, D.H.N.; Nguyen, T.H.; Vo, T.N.N.; Pham, L.P.T.; Vo, D.M.H.; Nguyen, C.K.; Bach, L.G.; Nguyen, D.H. Self-assembled poly (ethylene glycol) methyl ether-grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment. J. Appl. Polym. Sci. 2019, 136, 47544. [Google Scholar] [CrossRef] [Green Version]
- Camargo, L.E.A.d.; Brustolin Ludwig, D.; Tominaga, T.T.; Carletto, B.; Favero, G.M.; Mainardes, R.M.; Khalil, N.M. Bovine serum albumin nanoparticles improve the antitumour activity of curcumin in a murine melanoma model. J. Microencapsul. 2018, 35, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Meikle, T.G.; Su, Y.; Wang, X.; Dekiwadia, C.; Drummond, C.J.; Conn, C.E.; Yang, Y.J. Encapsulation in egg white protein nanoparticles protects anti-oxidant activity of curcumin. Food Chem. 2019, 280, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shih, F.-Y.; Su, I.-J.; Chu, L.-L.; Lin, X.; Kuo, S.-C.; Hou, Y.-C.; Chiang, Y.-T. Development of pectin-type B gelatin polyelectrolyte complex for curcumin delivery in anticancer therapy. Int. J. Mol. Sci. 2018, 19, 3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Controlled Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Park, K. Albumin: A versatile carrier for drug delivery. J. Controlled Release 2012, 1, 3. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and characterization of layer-by-layer composite nanoparticles based on zein and hyaluronic acid for co-delivery of curcumin and quercetagetin. ACS Appl. Mater. Int. 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Balaguer, M.; Gavara, R.; Hernandez-Munoz, P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocolloids 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Seok, H.-Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.-K.; Kim, Y.-C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Controlled Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocolloids 2020, 99, 105334. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Bao, H.; Liang, J.; Xu, S.; Cheng, G.; Zhu, Y. Fabrication and characterization of silk fibroin/curcumin sustained-release film. Materials 2019, 12, 3340. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-silk core–shell nanoparticles as potential carriers for targeted delivery of curcumin into human breast cancer cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Yu, J.; Zhao, Z.; Zheng, Z.; Xie, M.; Wang, X.; Han, Z.; Li, G. Fabrication and drug release properties of curcumin-loaded silk fibroin nanofibrous membranes. Adsorpt. Sci. Technol. 2019, 37, 412–424. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of stable and self-assembling rapeseed protein nanogel for hydrophobic curcumin delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef]
- Kadam, D.; Palamthodi, S.; Lele, S. Complexation of curcumin with Lepidium sativum protein hydrolysate as a novel curcumin delivery system. Food Chem. 2019, 298, 125091. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, Q.-T.; Zhu, J.; Li, X.-M.; Meng, R.; Zhang, B.; Chen, H.-Q.; Jin, Z.-Y. Study on the fabrication and in vitro digestion behavior of curcumin-loaded emulsions stabilized by succinylated whey protein hydrolysates. Food Chem. 2019, 287, 76–84. [Google Scholar] [CrossRef]
- Weng, Q.; Cai, X.; Zhang, F.; Wang, S. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of curcumin. Food Chem. 2019, 274, 796–802. [Google Scholar] [CrossRef]
- Wang, L.; Gulati, P.; Santra, D.; Rose, D.; Zhang, Y. Nanoparticles prepared by proso millet protein as novel curcumin delivery system. Food Chem. 2018, 240, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, R.; Eslahi, N.; Tamjid, E.; Simchi, A.A. Dual-sensitive hydrogel nanoparticles based on conjugated thermoresponsive copolymers and protein filaments for triggerable drug delivery. ACS Appl. Mater. Int. 2018, 10, 19336–19346. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mejia, L.M.; Meiser, M.; Rouf, T.; Hua, Y.; Kokini, J. Development of hollow kafirin-based nanoparticles fabricated through layer-by-layer assembly as delivery vehicles for curcumin. Food Hydrocolloids 2019, 96, 93–101. [Google Scholar] [CrossRef]
- Hassanzadeh Davarania, F.; Ashrafizadeh, M.; Saberi Riseh, R. Antifungal nanoparticles reduce aflatoxin contamination in pistachio. PHJ 2018, 1, 25–33. [Google Scholar]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef]
- Mohammadi, Z.A.; Aghamiri, S.F.; Zarrabi, A.; Talaie, M.R. A comparative study on non-covalent functionalization of carbon nanotubes by chitosan and its derivatives for delivery of doxorubicin. Chem. Phys. Lett. 2015, 642, 22–28. [Google Scholar] [CrossRef]
- Vijayakurup, V.; Thulasidasan, A.T.; Retnakumari, A.P.; Nandan, C.D.; Somaraj, J.; Antony, J.; Alex, V.V.; Vinod, B.S.; Liju, V.B.; Sundaram, S. Chitosan Encapsulation Enhances the Bioavailability and Tissue Retention of Curcumin and Improves its Efficacy in Preventing B [a] P-induced Lung Carcinogenesis. Cancer Prev. Res. 2019, 12, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi Nasab, N.; Hassani Kumleh, H.; Beygzadeh, M.; Teimourian, S.; Kazemzad, M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–81. [Google Scholar] [CrossRef]
- Razi, M.A.; Wakabayashi, R.; Tahara, Y.; Goto, M.; Kamiya, N. Genipin-stabilized caseinate-chitosan nanoparticles for enhanced stability and anti-cancer activity of curcumin. Colloids Surf. B. Biointerfaces 2018, 164, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Jia, X.; Zhao, X.; Li, J.; Liu, P. Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohydr. Polym. 2018, 183, 131–139. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Controlled Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Villa, C.C.; Sanchez, L.T.; Rodriguez-Marin, N.D. Starch nanoparticles and nanocrystals as bioactive molecule carriers. In Polymers for Agri-Food Applications; Tomy, J.G., Ed.; Springer: Berlin, Germany, 2019; pp. 91–98. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Weng, Z.; et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.P.; Biswal, S.; Nayak, L. Preparation of starch-chitosan nanocomposites for control drug release of curcumin. Int. J. Curr. Eng. Technol. 2015, 5, 336–343. [Google Scholar]
- Jahanizadeh, S.; Yazdian, F.; Marjani, A.; Omidi, M.; Rashedi, H. Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. Int. J. Biol. Macromol. 2017, 105, 757–763. [Google Scholar] [CrossRef]
- Athira, G.K.; Jyothi, A.N. Cassava starch-poly (vinyl alcohol) nanocomposites for the controlled delivery of curcumin in cancer prevention and treatment. Starch-Stärke 2015, 67, 549–558. [Google Scholar] [CrossRef]
- Li, X.-M.; Wu, Z.-Z.; Zhang, B.; Pan, Y.; Meng, R.; Chen, H.-Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019, 293, 197–203. [Google Scholar] [CrossRef]
- Abou-Saleh, R.H.; Hernandez-Gomez, M.C.; Amsbury, S.; Paniagua, C.; Bourdon, M.; Miyashima, S.; Helariutta, Y.; Fuller, M.; Budtova, T.; Connell, S.D. Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures. Nat. Commun. 2018, 9, 4538. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef] [PubMed]
- Kanagarajan, S.V.; Thiyagarajan, D. Carboxymethyl cellulose-functionalised magnetic nanocarriers for pH responsive delivery of Curcumin in cancer therapy. Mater. Res. Express 2018, 6, 016105. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Guo, Z.; Ju, Q.; Zhu, L.; Gao, J.; Zhou, L.; Liu, F.; Xu, Y.; Zhan, Q. A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Sci. Rep. 2016, 6, 28083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomer, A.; Vendrig, T.; Hopmans, E.S.; van Eijndhoven, M.; Middeldorp, J.M.; Pegtel, D.M. Exosomes: Fit to deliver small RNA. Commun. Int. Biol. 2010, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ji, Q.; Yang, Y.; Li, Q.; Wang, Z. Exosome: Function and role in cancer metastasis and drug resistance. Technol. Cancer Res. Treat. 2018. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.-J.; Sun, X.-Y.; Huang, K.-M.; Zhang, L.; Yang, Z.-S.; Zou, D.-D.; Wang, B.; Warnock, G.L.; Dai, L.-J.; Luo, J. Therapeutic potential of CAR-T cell-derived exosomes: A cell-free modality for targeted cancer therapy. Oncotarget 2015, 6, 44179. [Google Scholar] [CrossRef] [Green Version]
- Bang, C.; Thum, T. Exosomes: New players in cell–cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Delivery Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef]
- Hood, J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef] [Green Version]
- Goh, W.J.; Zou, S.; Ong, W.Y.; Torta, F.; Alexandra, A.F.; Schiffelers, R.M.; Storm, G.; Wang, J.-W.; Czarny, B.; Pastorin, G. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: A cost-effective alternative. Sci. Rep. 2017, 7, 14322. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.-P.; Goh, B.-C. Exosome-mediated metastasis: From epithelial–mesenchymal transition to escape from immunosurveillance. Trends Pharm. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of tumor-derived exosomes in cancer metastasis. Biochim. Biophys. Acta-Rev. Cancer 2019, 1871, 12–19. [Google Scholar] [CrossRef]
- Sun, L.; He, Q.; Qin, Z.; Lei, J.; Feng, B. Exosome and its applications as a novel drug delivery system. Clin. Oncol. 2017, 2, 1346. [Google Scholar]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Van der Meel, R.; Fens, M.H.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Controlled Release 2014, 195, 72–85. [Google Scholar] [CrossRef]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Controlled Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhou, J.; Zeng, C.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Curcumin increases exosomal TCF21 thus suppressing exosome-induced lung cancer. Oncotarget 2016, 7, 87081. [Google Scholar] [CrossRef] [Green Version]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P. Curcumin-primed and curcumin-loaded exosomes: Potential neural therapy. Neural Regen. Res. 2017, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Kang, Y.Y.; Mok, H. Evaluation of the enhanced antioxidant activity of curcumin within exosomes by fluorescence monitoring. Biotechnol. Bioprocess Eng. 2018, 23, 150–157. [Google Scholar] [CrossRef]
- Letchford, K.; Liggins, R.; Burt, H. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: Theoretical and experimental data and correlations. J. Pharm. Sci. 2008, 97, 1179–1190. [Google Scholar] [CrossRef]
- Yoncheva, K.; Kamenova, K.; Perperieva, T.; Hadjimitova, V.; Donchev, P.; Kaloyanov, K.; Konstantinov, S.; Kondeva-Burdina, M.; Tzankova, V.; Petrov, P. Cationic triblock copolymer micelles enhance antioxidant activity, intracellular uptake and cytotoxicity of curcumin. Int. J. Pharm. 2015, 490, 298–307. [Google Scholar] [CrossRef]
- Petrov, P.D.; Yoncheva, K.; Gancheva, V.; Konstantinov, S.; Trzebicka, B. Multifunctional block copolymer nanocarriers for co-delivery of silver nanoparticles and curcumin: Synthesis and enhanced efficacy against tumor cells. Eur. Polym. J. 2016, 81, 24–33. [Google Scholar] [CrossRef]
- Wittemann, A.; Azzam, T.; Eisenberg, A. Biocompatible polymer vesicles from biamphiphilic triblock copolymers and their interaction with bovine serum albumin. Langmuir 2007, 23, 2224–2230. [Google Scholar] [CrossRef]
- Zhang, W.; He, J.; Liu, Z.; Ni, P.; Zhu, X. Biocompatible and pH-responsive triblock copolymer mPEG-b-PCL-b-PDMAEMA: Synthesis, self-assembly, and application. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1079–1091. [Google Scholar] [CrossRef]
- Mazzarino, L.; Otsuka, I.; Halila, S.; Bubniak Ldos, S.; Mazzucco, S.; Santos-Silva, M.C.; Lemos-Senna, E.; Borsali, R. Xyloglucan-block-poly(-caprolactone) copolymer nanoparticles coated with chitosan as biocompatible mucoadhesive drug delivery system. Macromol. Biosci. 2014, 14, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Mirakabad, F.S.; Akbarzadeh, A.; Milani, M.; Zarghami, N.; Taheri-Anganeh, M.; Zeighamian, V.; Badrzadeh, F.; Rahmati-Yamchi, M. A Comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hu, X.; Szymusiak, M.; Wang, Z.J.; Liu, Y. Orally administered nanocurcumin to attenuate morphine tolerance: Comparison between negatively charged PLGA and partially and fully PEGylated nanoparticles. Mol. Pharm. 2013, 10, 4546–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Lu, Y.; Zhang, X.; Wang, H.; Han, J.; Dong, C. Novel curcumin-loaded human serum albumin nanoparticles surface functionalized with folate: Characterization and in vitro/vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 2643. [Google Scholar]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q10 as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733. [Google Scholar]
- Vrána, A.; Andrysek, T. The effect of particle size on bioavailability in cyclosporine preparations based on submicron dispersions. Biomed. Pap.-Palacky Univ. Olomouc 2001, 145, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, L. Lipid–polymer hybrid nanoparticles: Synthesis, characterization and applications. Nano. Life 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef] [Green Version]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Martin, R.C.; Locatelli, E.; Li, Y.; Zhang, W.; Li, S.; Monaco, I.; Franchini, M.C. Gold nanorods and curcumin-loaded nanomicelles for efficient in vivo photothermal therapy of Barrett’s esophagus. Nanomedicine 2015, 10, 1723–1733. [Google Scholar] [CrossRef]
- Nosrati, H.; Charmi, J.; Salehiabar, M.; Abhari, F.; Danafar, H. Tumor targeted albumin coated bismuth sulfide nanoparticles (Bi2S3) as radiosensitizers and carriers of curcumin for enhanced chemoradiation therapy. ACS Biomater. Sci. Eng. 2019, 5, 4416–4424. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Huang, S.; Xu, W.; Wan, J.; Wang, D.; Zheng, G.; Xia, Z. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int. J. Nanomed. 2018, 13, 8309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Su, X.; Gregory, D.; Li, W.; Cai, Z.; Zhao, X. Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Evaluation of folic acid tagged aminated starch/ZnO coated iron oxide nanoparticles as targeted curcumin delivery system. Carbohydr. Polym. 2017, 157, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Int. Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Thulasidasan, A.K.T.; Retnakumari, A.P.; Shankar, M.; Vijayakurup, V.; Anwar, S.; Thankachan, S.; Pillai, K.S.; Pillai, J.J.; Nandan, C.D.; Alex, V.V. Folic acid conjugation improves the bioavailability and chemosensitizing efficacy of curcumin-encapsulated PLGA-PEG nanoparticles towards paclitaxel chemotherapy. Oncotarget 2017, 8, 107374. [Google Scholar] [CrossRef] [Green Version]
- Nam, N.H.; Doan, D.H.; Nhung, H.T.M.; Quang, B.T.; Nam, P.H.; Thong, P.Q.; Phuc, N.X.; Thu, H.P. Folate attached, curcumin loaded Fe3O4 nanoparticles: A novel multifunctional drug delivery system for cancer treatment. Mater. Chem. Phys. 2016, 172, 98–104. [Google Scholar]
- Li, L.; Xiang, D.; Shigdar, S.; Yang, W.; Li, Q.; Lin, J.; Liu, K.; Duan, W. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int. J. Nanomed. 2014, 9, 1083–1096. [Google Scholar]
| Polymer | Size | Zeta Potential | LC or EE | Cell Line/Animal Model | Advantages | Refs. |
|---|---|---|---|---|---|---|
| BSA@CUR NPs | 92.59 ± 16.75 nm | −9.19 mV | 18.3% | MCF-7 cells | Increased therapeutic efficacy | [77] |
| Curcumin in BSA-dextran NP | 115 nm | 2.8% | Caco-2 cells | Better stability Improve the cellular antioxidant activity of curcumin | [91] | |
| Curcumin cross-linked HSA NPs | 125 nm | −12.36 ± 0.73 to −10.88 ± 0.6 mV | was dependent on the particle size | A549 cells | Improved cellular uptake Increased the cytotoxicity | [92] |
| Curcumin-loaded zein NPs | 66 nm | +17.1 mV | 7.3 ± 0.1% | GIT model | May be useful for application in functional foods or beverages | [93] |
| Curcumin-zein/rhamnolipid complex | 77.29 nm | −31 mV To +3 mV | EE: 98.05% | In vitro simulated gastrointestinal tract | Protect hydrophobic bioactive compounds | [94] |
| Pectin-coated CZ NPs | 250 nm to 600 nm | −45 to −50 mV | 5% | Simulated gastrointestinal digestive condition | Enhanced antioxidant activity in an aqueous environment | [95] |
| Curcumin-loaded zein NPs with (SC) and (SA) | 190 nm | 17 mV to 19.8 mV | EE: 36.10% to 76.06% | Improving the water solubility Improving photochemical stability improving antioxidant activity | [96] | |
| Curcumin-loaded silk fibroin NPs | 155 nm to 170 nm | −45 mV | EE: 50% | Kelly Cells | Higher efficacy in cytotoxicity | [7] |
| Curcumin plus SFNs | 71 ± 10 nm | 1.50 ± 0.11 to 11.40 ± 0.76 | In vitro model of osteoarthritis | Exhibited a synergistic antioxidant effect Improve cyto- and hemo-compatibility | [97] | |
| CUR-loaded silk NPs | 229 nm to 2286 nm | −17.8 nm to −18.9 mV | 22 to 41% | Rats | Longer plasma circulation time | [98] |
| CUR Loaded RBA−CS NPs | 778 nm | Negative | EE: 93.56% | Caco-2 cells | A great potential application for hydrophobic active agent delivery | [99] |
| Zein-HA NPs | 186.4 nm | –35.2 to −28.7 mV | 3.66% | Simulate gastrointestinal digestion | Better stability of anti-light degradation, and control release | [100] |
| SSPS NPs | 200 nm to 300 nm | EE: 90% | HCT116 and MCF-7 cells | Improved activity Improvement in the anti-proliferative activity | [101] | |
| Cur-ACRU/CS NPs | 200 nm to 450 nm | +15 mV | 5.4% | Caco-2 cells | Improved permeability efficiency of free curcumin | [102] |
| Cur-Chitosan NPs | 167 nm to 251 nm | + 18.1 to + 20.2 mV | EE: 80% | HaCaT cells | Superior drug release Enhanced transdermal permeation of curcumin A superior percentage of cell viability | [103] |
| CDG-CANPs | 215 nm | −24.1 mV | 27% | Caco-2 cells | Improvement of physicochemical stabilities, digestibility, bioaccessibility and cellular uptake | [104] |
| CUR-AlgNP | 100-600 nm | −36.0± 0.4 | EE: 68.3% | HeLa and H9c2 | Kills the cancer cell lines at lower concentrations | [105] |
| Cur-CS/Alg NPs | 199 nm to 1120 nm | −30.8 mV to −10.8 mV | 0% to 27.4% | HaCaT cells | Improved the cellular uptake of curcumin | [106] |
| Starch NPs | < 250 nm | −30 mV | EE: 80% | Simulated gastric and intestinal fluids | Higher encapsulation efficiency | [107] |
| OSA starch loaded nano curcumin | 10 nm to 50 nm | HeLa cells | Anti-cancer potential Significant enhancement in cellular uptake Increase bioavailability More controlled release | [108] | ||
| Curcumin-load film | 159 ± 31 nm in length and 2 nm in width. | Rat | Improved the regeneration of hair follicles And sebaceous glands of the skin Attenuated the bacterial growth | [109] | ||
| Cur- NLCs | 500 nm | EE~58.8 ± 3.5 | Mouse | Reducing the pro-inflammatory cytokine levels in the skin | [110] | |
| ANC NPs | ≤150 nm | -31.2 ± 3.66 mV | EE > 90% | L929 and MCF-7 cells | Inhibit microbial growth Prevent preferential killing of cancer cells compared to normal cells | [111] |
| WPI-Lac/EGCG NPs | 110 nm | 27 mV | Better protective effect on the breakdown of curcumin in Pickering emulsions More even droplet distribution Greater thermal stability Higher curcumin percentage retention | [112] | ||
| CUR-Loaded Gel-mPEG Nanogels | 147 ± 5.2 nm | −12.8 ± 0.6 | 7.9 ± 0.2%, | HeLa cells | Improved solubility Enhanced therapeutic efficacy | [113] |
| Curcumin-loaded BSA NPs | 150 nm | Negative | EE: 45% | Murine melanoma model | Increase in survival rate associated with a reduction in tumor size | [114] |
| Curcumin loading EWP | 59.25 nm to 431.3 nm | >+30 mV | 11.2 mg/g | Protect the antioxidant activity of encapsulated curcumin | [115] | |
| Curcumin-PECs | 264.0 ± 3.1 nm | EE: 53% | HCT116 cells | Induced cell cycle arrest Exhibited cytotoxic effect | [116] |
| Polymer | The Route of Targeting | Size | Zeta Potential | LC or EE | Cell Line/Animal Model | Advantages | Refs. |
|---|---|---|---|---|---|---|---|
| F-CUR-HSANPs | Folate | 165.6 ± 15.7 nm | −27.3 ± 4.2 mV | EE:88.7% ± 4.8% | Murine colon cancer model | Maintained sustained release, and a faster release of CM compare to the unconjugated NPs | [188] |
| Apt-HSA/CCM NP | Aptamer to target HER-2 positive cells | 281.1 nm | −33.3 ± 2.5mV | 3.4% | SK-BR3 cells | Higher toxicity | [120] |
| Gal-BSA-Cur NPs | Galactosylation to target asialoglycoprotein receptor (ASGPR) overexpressed on hepatocellular carcinoma (HCC) cells | 116.24 nm | −14.12 ± 1.81 | EE:55.47% ± 0.45% | HCC cell line | Enhanced the internalization ability of drug compared with BSA NPs-loaded curcumin | [196] |
| Zein and HA for the co-delivery of curcumin and quercetagetin | HA | 231 nm | −30.5 mV | 2.5% | simulated gastrointestinal tract conditions | Improve oral bioavailability | [122] |
| Curcumin loaded magnetic silk fibroin core–shell NPs | Magnetic NP | 30 nm to 250 nm | LC: 8.4% | MDA-MB-231 cells | Enhanced growth inhibition | [128] | |
| Bi2S3@BSA-FA-CUR | Folic acid | 170.9 nm | −23.2 mV | LC:10 ± 1.51% | The mouse breast carcinoma cell line, Murine breast cancer model | Enhanced the efficacy of chemoradiation therapy | [195] |
| magnetic alginate/chitosan layer-by-layer nanoparticles (MACPs) | Fe3O4 NPs | 172 nm to 199 nm | EE: 49.2% | MDA-MB-231 breast cancer cells, HDF cells | The sustained release profiles, enhanced uptake efficiency and cytotoxicity to cancer cells | [197] | |
| folic acid tagged aminated starch/ZnO coated iron oxide nanoparticles as targeted curcumin delivery system | Fe3O4 NPs | 31.2 ± 2 | 42.9 ± 0.03 | EE: 76.8 ± 0.04% | HepG2 and MCF7 cell lines | Enhanced the uptake by HepG2 cells | [198] |
| Cur loaded MnFe2O4–CMC | Fe2O4 NPs | 35 nm | MCF7 and HeLa cells | Enhanced the therapeutic efficacy | [156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030689
Moballegh Nasery M, Abadi B, Poormoghadam D, Zarrabi A, Keyhanvar P, Khanbabaei H, Ashrafizadeh M, Mohammadinejad R, Tavakol S, Sethi G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules. 2020; 25(3):689. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030689
Chicago/Turabian StyleMoballegh Nasery, Mahshid, Banafshe Abadi, Delaram Poormoghadam, Ali Zarrabi, Peyman Keyhanvar, Hashem Khanbabaei, Milad Ashrafizadeh, Reza Mohammadinejad, Shima Tavakol, and Gautam Sethi. 2020. "Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review" Molecules 25, no. 3: 689. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030689