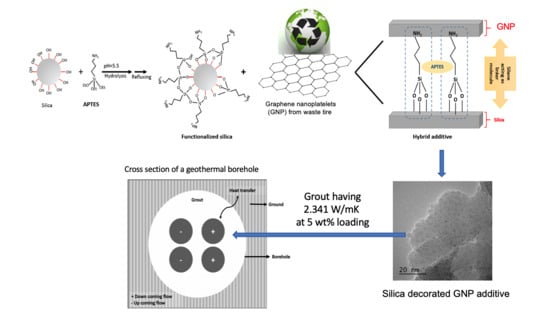
Facile Synthesis of Graphene from Waste Tire/Silica Hybrid Additives and Optimization Study for the Fabrication of Thermally Enhanced Cement Grouts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization Study for Surface Functionalization of Silica
2.2. Morphological and Structural Properties of Silica-GNP Hybrid Additive
2.3. Grout Formulations by GNP Based Hybrid Additives and Their Characteristics
3. Materials and Methods
3.1. Materials
3.2. Method of Surface Functionalization of Silica
3.3. Hybridization of Functionalized Silica with GNP
3.4. Preparation of Grouts by the Addition of Si-GNP Hybrid Additives
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allan, M.L.; Kavanaugh, S.P. Thermal conductivity of cementitious grouts and impact on heat exchanger length design for ground source heat pumps. HVAC R Res. 1999, 5, 85–96. [Google Scholar] [CrossRef]
- Javadi, H.; Mousavi Ajarostaghi, S.; Rosen, M.A.; Pourfallah, M. A comprehensive review of backfill materials and their effects on ground heat exchanger performance. Sustainability 2018, 10, 4486. [Google Scholar] [CrossRef] [Green Version]
- Thermal Cracking of Concrete WHY Does Thermal Cracking Occur? Concrete in Practice. Available online: https://www.nrmca.org/aboutconcrete/cips/42p.pdf (accessed on 17 February 2020).
- Sedaghat, A.; Ram, M.K.; Zayed, A.; Kamal, R.; Shanahan, N. Investigation of Physical Properties of Graphene-Cement Composite for Structural Applications. Open J. Compos. Mater. 2014, 4, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ouyang, D. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials 2017, 7, 187. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J. Effects of various carbon additives on the thermal storage performance of form-stable PCM integrated cementitious composites. Appl. Therm. Eng. 2019, 148, 491–501. [Google Scholar] [CrossRef]
- Habsya, C.; Diharjo, K.; Setyono, P.; Satwiko, P. Physical, mechanical and thermal properties of lightweight foamed concrete with fly ash. IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012062. [Google Scholar] [CrossRef] [Green Version]
- Asadi, I.; Shafigh, P.; Hassan, Z.F.B.A.; Mahyuddin, N.B. Thermal conductivity of concrete—A review. J. Build. Eng. 2018, 20, 81–93. [Google Scholar] [CrossRef]
- Jobmann, M.; Buntebarth, G. Influence of graphite and quartz addition on the thermo—Physical properties of bentonite for sealing heat-generating radioactive waste. Appl. Clay Sci. 2009, 44, 206–210. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Fundamental interaction of hydrophobic materials in concrete with different moisture contents in saline environment. Constr. Build. Mater. 2019, 207, 122–135. [Google Scholar] [CrossRef]
- Krasnoslobodtsev, A.V.; Smirnov, S.N. Effect of water on silanization of silica by trimethoxysilanes. Langmuir 2002, 18, 3181–3184. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Nascimento, A.J.P.; De Oliveira, F.J.R.; Bruns, R.E.; Airoldi, C. New factorial designs to evaluate chemisorption of divalent metals on aminated silicas. J. Colloid Interface Sci. 2001, 241, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, E.T.; Bertilsson, L.; Liedberg, B.; Uvdal, K.; Erlandsson, R.; Elwing, H.; Lundström, I. Structure of 3-aminopropyl triethoxy silane on silicon oxide. J. Colloid Interface Sci. 1991, 147, 103–118. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.Y.; Pan, S.; Guo, Z.W. Synthesis and properties of a silane and copolymer-modified graphene oxide for use as a water-reducing agent in cement pastes. New Carbon Mater. 2018, 33, 131–139. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Liu, Y.; Ge, C.; Guo, L.; Shu, X.; Liu, J. Synergistic effects of silica nanoparticles/polycarboxylate superplasticizer modified graphene oxide on mechanical behavior and hydration process of cement composites. RSC Adv. 2017, 7, 16688–16702. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, C.E.; Kupwade-Patil, K.; Ortega, M.; Soriano, C.; Büyüköztürk, O.; White, A.E.; Short, M.P. Irradiated recycled plastic as a concrete additive for improved chemo-mechanical properties and lower carbon footprint. Waste Manag. 2018, 71, 426–439. [Google Scholar] [CrossRef]
- Norambuena-Contreras, J.; Thomas, C.; Borinaga-Treviño, R.; Lombillo, I. Influence of recycled carbon powder waste addition on the physical and mechanical properties of cement pastes. Mater. Struct. Constr. 2016, 49, 5147–5159. [Google Scholar] [CrossRef]
- Zareei, S.A.; Ameri, F.; Dorostkar, F.; Ahmadi, M. Rice husk ash as a partial replacement of cement in high strength concrete containing micro silica: Evaluating durability and mechanical properties. Case Stud. Constr. Mater. 2017, 7, 73–81. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. Available online: http://0-www-jstor-org.brum.beds.ac.uk/stable/108699 (accessed on 27 April 2019).
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1899, 32, 1394–1399. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kluth, G.J.; Sung, M.M.; Maboudian, R. Thermal behavior of alkylsiloxane self-assembled monolayers on the oxidized Si(100) surface. Langmuir 1997, 13, 3775–3780. [Google Scholar] [CrossRef]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Snow, L.D.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3-(aminopropyl)triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef]
- Pena, A.R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of c-Aminopropyltriethoxysilane by FT-IR spectroscopy. J. Mater. Sci. 2007, 42, 595–603. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Sehlleier, Y.H.; Abdali, A.; Schnurre, S.M.; Wiggers, H.; Schulz, C. Surface functionalization of microwave plasma-synthesized silica nanoparticles for enhancing the stability of dispersions. J. Nanopart. Res. 2014, 16, 2557. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Kim, D.S.; Dhand, V.; Rhee, K.Y.; Park, S.J. Study on the effect of silanization and improvement in the tensile behavior of graphene-chitosan-composite. Polymers 2015, 7, 527–551. [Google Scholar] [CrossRef] [Green Version]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Williams, E.H.; Schreifels, J.A.; Rao, M.V.; Davydov, A.V.; Oleshko, V.P.; Lin, N.J.; Steffens, K.L.; Krylyuk, S.; Bertness, K.A.; Manocchi, A.K.; et al. Selective streptavidin bioconjugation on silicon and silicon carbide nanowires for biosensor applications. J. Mater. Res. 2013, 28, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Haghighi Poudeh, L.; Cakiroglu, D.; Cebeci, F.Ç.; Yildiz, M.; Menceloglu, Y.Z.; Saner Okan, B. Design of Pt-Supported 1D and 3D Multilayer Graphene-Based Structural Composite Electrodes with Controlled Morphology by Core-Shell Electrospinning/Electrospraying. ACS Omega 2018, 3, 6400–6410. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Wen, X.; Jiang, Z.; Chen, X.; Mijowska, E.; Tang, T. Upcycling waste polypropylene into graphene flakes on organically modified montmorillonite. Ind. Eng. Chem. Res. 2014, 53, 4173–4181. [Google Scholar] [CrossRef]
- Reuss, M.; Proell, M.; Koenigsdorff, R. Quality Control of Borehole Heat Exchanger Systems. In Proceedings of the Effstock’09 Conference, Stockholm, Sweden, 14–17 June 2009. [Google Scholar]
- Javed, S.; Spitler, J.D. Calculation of Borehole Thermal Resistance; Aalborg, Denmark. In Advances in Ground-Source Heat Pump Systems; Woodhead Publishing: Chichester, UK, 2016; Volume 1, pp. 63–95. ISBN 9780081003220. [Google Scholar]
- Chiasson, A.D. Theory and practice. In Geothermal Heatpump and Heatengine Systems; John Wiley & Sons: Chichester, UK, 2016; ISBN 9781118961971. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |










| Samples | Carbon (at%) | Oxygen (at%) | Silicon (at%) | Nitrogen (at%) | Other (at%) |
|---|---|---|---|---|---|
| Silica:APTES = 1:2 | 26 | 43 | 27 | 3.1 | - |
| GNP | 87 | 9 | 2 | - | 2 |
| Si:GNP = 1:5 | 53 | 30 | 16 | 1 | - |
| Si:GNP = 1:10 | 60 | 25.1 | 12.7 | 1.5 | 0.7 |
| Test Number | Cement (gr) | Silica Sand 30–35 AFS (gr) | Silica Sand 60–70 AFS (gr) | Bentonite (gr) | Additive (gr) | SP (gr) | Water (gr) | Marshcone (sec) | Flowtable (cm) | Bleeding (%) | Density (g·cm−3) | Thermal Conductivity (W·m−1K−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 930 | 900 | 900 | 10 | 0 Reference | 18.6 | 650 | 77 | 26 | 0.49 | 2.1 | 2.373 |
| 2 | 930 | 900 | 900 | 10 | 9.3 (Si:GNP = 1:5) (1 wt%) | 18.6 | 650 | 90 | 28 | <0.3 | 2.02 | 2.427 |
| 3 | 930 | 900 | 900 | 10 | 27.9 (Si:GNP = 1:5) (3 wt%) | 18.6 | 660 | 105 | 24 | 0.10 | 2.04 | 2.287 |
| 4 | 930 | 900 | 900 | 10 | 46.5 (Si:GNP = 1:5) (5 wt%) | 18.6 | 725 | 95 | 27 | 0.25 | 2.03 | 1.816 |
| 5 | 930 | 900 | 900 | 10 | 46.5 (Si:GNP = 1:10) (5 wt%) | 18.6 | 700 | 96 | 28 | 1.2 | 2.05 | 2.341 |
| Sample | Silica Amount (g) | APTES Amount (mL) | Reaction Time (h) | Reaction Medium | Reaction Temperature (°C) |
|---|---|---|---|---|---|
| Si:APTES = 1:1 | 1 | 1 | 24 | Water | 80 |
| Si:APTES = 1:2 | 1 | 2 | 24 | Water | 80 |
| Si:APTES = 1:3 | 1 | 3 | 24 | Water | 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berktas, I.; Ghafar, A.N.; Fontana, P.; Caputcu, A.; Menceloglu, Y.; Okan, B.S. Facile Synthesis of Graphene from Waste Tire/Silica Hybrid Additives and Optimization Study for the Fabrication of Thermally Enhanced Cement Grouts. Molecules 2020, 25, 886. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040886
Berktas I, Ghafar AN, Fontana P, Caputcu A, Menceloglu Y, Okan BS. Facile Synthesis of Graphene from Waste Tire/Silica Hybrid Additives and Optimization Study for the Fabrication of Thermally Enhanced Cement Grouts. Molecules. 2020; 25(4):886. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040886
Chicago/Turabian StyleBerktas, Ilayda, Ali Nejad Ghafar, Patrick Fontana, Ayten Caputcu, Yusuf Menceloglu, and Burcu Saner Okan. 2020. "Facile Synthesis of Graphene from Waste Tire/Silica Hybrid Additives and Optimization Study for the Fabrication of Thermally Enhanced Cement Grouts" Molecules 25, no. 4: 886. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25040886