Direct Dehydrative Glycosylation Catalyzed by Diphenylammonium Triflate
Abstract
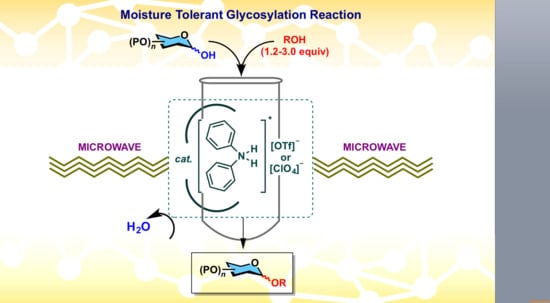
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Demchenko, A.V. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 2008. [Google Scholar]
- Gin, D. Dehydrative glycosylation with 1-hydroxy donors. J. Carbohydr. Chem. 2002, 21, 645–665. [Google Scholar] [CrossRef]
- Kim, K.S.; Fulse, D.B.; Baek, J.Y.; Lee, B.-Y.; Jeon, H.B. Stereoselective direct glycosylation with anomeric hydroxy sugars by activation with phthalic anhydride and trifluoromethanesulfonic anhydride involving glycosyl phthalate intermediates. J. Am. Chem. Soc. 2008, 130, 8537–8547. [Google Scholar] [CrossRef]
- Chen, G.; Yin, Q.; Yin, J.; Gu, X.; Liu, X.; You, Q.; Chen, Y.-L.; Xiong, B.; Shen, J. Strained olefin enables triflic anhydride mediated direct dehydrative glycosylation. Org. Biomol. Chem. 2014, 12, 9781–9785. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, K.A.; Taylor, M.S. Borinic acid catalyzed stereo- and regioselective couplings of glycosyl methanesulfonates. J. Am. Chem. Soc. 2016, 138, 11058–11066. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.M.; Bylsma, M.; Bright, D.K.; Bennett, C.S. Reagent-controlled α-selective dehydrative glycosylation of 2,6-dideoxy- and 2,3,6-trideoxy sugars. Angew. Chem., Int. Ed. 2016, 55, 10088–10092. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Rodriguez, J.; Walczak, M.A. Direct dehydrative glycosylation of C1-alcohols. Chem.Asian J. 2018, 13, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E. Ueber die glucoside der alkohole. Ber. Dtsch. Chem. Ges. 1893, 26, 2400–2412. [Google Scholar] [CrossRef] [Green Version]
- Naohiro, A.; Shu, K. Dehydrative glycosylation in water using a Brønsted acid–surfactant-combined catalyst. Chem. Lett. 2006, 35, 238–239. [Google Scholar]
- Park, T.-J.; Weïwer, M.; Yuan, X.; Baytas, S.N.; Munoz, E.M.; Murugesan, S.; Linhardt, R.J. Glycosylation in room temperature ionic liquid using unprotected and unactivated donors. Carbohydr. Res. 2007, 342, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Mukherji, A.; Srivastava, H.K.; Kancharla, P.K. Secondary amine salt catalyzed controlled activation of 2-deoxy sugar lactols towards a-selective dehydrative glycosylation. Org. Biomol. Chem. 2018, 16, 2870–2875. [Google Scholar] [CrossRef]
- Ghosh, T.; Mukherji, A.; Kancharla, P.K. Open-close strategy toward the organocatalytic generation of 2-deoxyribosyl oxocarbenium ions: Pyrrolidine-salt-catalyzed synthesis of 2-deoxyribofuranosides. Eur. J. Org. Chem. 2019, 2019, 7488–7498. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium (IV) salts. Science 2000, 290, 1140–1142. [Google Scholar] [CrossRef]
- Ishihara, K. Dehydrative condensation catalyses. Tetrahedron 2009, 65, 1085–1109. [Google Scholar] [CrossRef]
- Wakasugi, K.; Misaki, T.; Yamada, K.; Tanabe, Y. Diphenylammonium triflate (DPAT): Efficient catalyst for esterification of carboxylic acids and for transesterification of carboxylic esters with nearly equimolar amounts of alcohols. Tetrahedron Lett. 2000, 41, 5249–5252. [Google Scholar] [CrossRef]
- Funatomi, T.; Wakasugi, K.; Misaki, T.; Tanabe, Y. Pentafluorophenylammonium triflate (PFPAT): An efficient, practical, and cost-effective catalyst for esterification, thioesterification, transesterification, and macrolactone formation. Green Chem. 2006, 8, 1022–1027. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakagawa, S.; Sakakura, A. Bulky diarylammonium arenesulfonates as selective esterification catalysts. J. Am. Chem. Soc. 2005, 127, 4168–4169. [Google Scholar] [CrossRef]
- Sakakura, A.; Nakagawa, S.; Ishihara, K. Bulky diarylammonium arenesulfonates as mild and extremely active dehydrative ester condensation catalysts. Tetrahedron 2006, 62, 422–433. [Google Scholar] [CrossRef]
- Sakakura, A.; Watanabe, H.; Nakagawa, S.; Ishihara, K. Unusual rate acceleration in Brønsted acid catalyzed dehydration reactions: Local hydrophobic environment in aggregated N-(2,6-diphenylphenyl)-N-mesitylammonium pentafluorobenzenesulfonates. Chem. Asian J. 2007, 2, 477–483. [Google Scholar] [CrossRef]
- Sakakura, A.; Nakagawa, S.; Ishihara, K. Direct ester condensation catalyzed by bulky diarylammonium pentafluorobenzenesulfonates. Nat. Protoc. 2007, 2, 1746–1751. [Google Scholar] [CrossRef] [Green Version]
- Sakakura, A.; Koshikari, Y.; Akakura, M.; Ishihara, K. Hydrophobic N,N-diarylammonium pyrosulfates as dehydrative condensation catalysts under aqueous conditions. Org. Lett. 2012, 14, 30–33. [Google Scholar] [CrossRef]
- Guthrie, J.P. Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units. Can. J. Chem. 1978, 56, 2342–2354. [Google Scholar]
- Bentley, C.L.; Bond, A.M.; Hollenkamp, A.F.; Mahon, P.J.; Zhang, J. Electrochemical proton reduction and equilibrium acidity (pKa) in aprotic ionic liquids: Phenols, carboxylic acids, and sulfonic acids. J. Phys. Chem. C 2015, 119, 21840–21851. [Google Scholar] [CrossRef]
- Trummal, A.; Lipping, L.; Kaljurand, I.; Koppel, I.A.; Leito, I. Acidity of strong acids in water and dimethyl sulfoxide. J. Phys. Chem. A 2016, 120, 3663–3669. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Heneghan, M.; Schnabel, G.; Ernst, B. Catalytic glycosylation with rhodium(III)-triphos catalysts. Synlett 2003, 2003, 1303–1306. [Google Scholar] [CrossRef]
- Eller, S.; Collot, M.; Yin, J.; Hahm, H.S.; Seeberger, P.H. Automated solid-phase synthesis of chondroitin sulfate glycosaminoglycans. Angew. Chem. Int. Ed. 2013, 52, 5858–5861. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available All authors have read and agreed to the published version of the manuscript.. |




| Entry | Catalyst | Yield b | α:β c |
|---|---|---|---|
| 1 | - | NR d | - |
| 2 | [(Mes)2NH2][O3S(C6F5)] (3a) | 90% | 1:1 |
| 3 | Ph2NH2OTf (DPAP) (3b) | 90% | 1:1 |
| 4 e | Ph2NH2OTf (DPAP) (3b) | 83% | 1:1 |
| 5 f | Ph2NH2OTf (DPAP) (3b) | 9% g | 1:1 |
| 6 | Me2NH2OTf (3c) | NR | - |
| 7 | Bn2NH2OTf (3d) | NR | - |
| 8 | Ph2NH2OMs (3e) | 5%g | ND h |
| 9 | Ph2NH2O3SPh (3f) | 5%g | ND h |
| 10 | Ph2NH2OTs (3g) | 5%g | ND h |
| 11 | Ph2NH2ClO4 (3h) | 89% | 1:1 |
| 12 | TfOH | 36% i | 2:1 |

| Entry | Acceptor | Yield a | α:β b |
|---|---|---|---|
| 1 | benzyl alcohol (2b) | 4b 75% | 2:1 |
| 2 | 2-propenol (2c) | 4c 80% | 2:1 |
| 3 | isopropanol (2d) | 4d 74% | 2:1 |
| 4 | cyclohexanol (2e) | 4e 76% | 2:1 |
| 5 c | 2f | 4f 60% | 2:1 |
| 6 c | 2g | 4g 48% | 1:1 |
| 7 c,d,e | 2h | 4h 64% | 2:1 |

| Entry | Donor | Acceptor | T (°C) | Yield a | α:β b |
|---|---|---|---|---|---|
| 1 c | 5 | 2h | 70 | 9h 68% | 2:1 |
| 2 c | 5 | 2i | 70 | 9i 58% | 3:1 |
| 3 | 6 | 2d | 80 | 10d 71% | 1:2 |
| 4 | 6 | 2e | 80 | 10e 79% | 1:2 |
| 5 c | 6 | 2f | 80 | 10f 60% | β-only |
| 6 c | 6 | 2h | 70 | 10h 62% | β-only |
| 7 c | 6 | 2i | 70 | 10i 56% | β-only |
| 8 d | 6 | 2j | 80 | 10j 63% | β-only |
| 9 e | 7 | 2a | 80 | 11a 58% | 1:1 |
| 10 e | 7 | 2d | 80 | 11d 75% | 2:1 |
| 11 e | 7 | 2e | 80 | 11e 62% | 2:1 |
| 12 e | 7 | 2f | 100 | 11f 39% | 1:1 |
| 13 | 8 | 2a | 100 | 12a 64% f | 1:2 |
| 14 | 8 | 2d | 100 | 12d 75% f | 3:1 |
| 15 | 8 | 2e | 100 | 12e 71% | 6:1 |

| Entry | Donor | Acceptor | Yield a | α:β b |
|---|---|---|---|---|
| 1 c | 13 | 2a | 15a 95% | 2:1 |
| 2 d,e | 13 | 2d | 15d 82% | 2:1 |
| 3 | 13 | 2e | 15e 59% | 2:1 |
| 4 | 13 | 2f | 15f 56% | 2:1 |
| 5 c | 14 | 2a | 16a 90% | α only |
| 6 d,e | 14 | 2d | 16d 82% | α only |
| 7 | 14 | 2e | 16e 73% | α only |
| 8 | 14 | 2f | 16f 62% | α only |

| Entry | Donor | Acceptor | Yield a | α:β b |
|---|---|---|---|---|
| 1 | 17 | 2d | 19d 68% | 3:1 |
| 2 | 17 | 2e | 19e 60% | 3:1 |
| 3 | 17 | 2f | 19f 52% | 5:1 |
| 4 | 17 | 2k | 19k 51% | 4:1 |
| 5 | 18 | 2d | 20d 70% | 6:1 |
| 6 | 18 | 2e | 20e 73% | 6:1 |
| 7 | 18 | 2f | 20f 68% | 10:1 |
| 8 | 18 | 2k | 20k 51% | 6:1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-Y.; Lam, S.; Wu, C.-H.; Lin, M.-H.; Lin, S.-C.; Wang, C.-C. Direct Dehydrative Glycosylation Catalyzed by Diphenylammonium Triflate. Molecules 2020, 25, 1103. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051103
Hsu M-Y, Lam S, Wu C-H, Lin M-H, Lin S-C, Wang C-C. Direct Dehydrative Glycosylation Catalyzed by Diphenylammonium Triflate. Molecules. 2020; 25(5):1103. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051103
Chicago/Turabian StyleHsu, Mei-Yuan, Sarah Lam, Chia-Hui Wu, Mei-Huei Lin, Su-Ching Lin, and Cheng-Chung Wang. 2020. "Direct Dehydrative Glycosylation Catalyzed by Diphenylammonium Triflate" Molecules 25, no. 5: 1103. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051103