New Selective Progesterone Receptor Modulators and Their Impact on the RANK/RANKL Complex Activity
Abstract
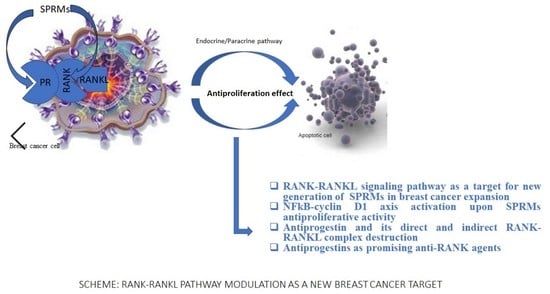
:1. Introduction
2. Results and Discussion
2.1. Proliferation Assay
2.2. NFkB Expression Assay
2.3. Cyclin D1 Expression Assay
2.4. GST-Pull Down Analysis of RANK-RANKL Complex Destabilization
3. Materials and Methods
3.1. Tested Compounds
3.2. Reagents
3.3. Cell Culture
3.4. The proliferation Assay
3.5. Estimation of NFkB Expression
3.5.1. Cell Test System
3.5.2. Transactivation Assay
3.6. Flow Cytometry Analysis of Cyclin D1 Expression
Cell Test System
3.7. GST-Pull Down Assay for RANK/RANKL Complex Binding
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jassem, J.; Krzakowski, M.; Bobek-Billewicz, B.; Duchnowska, R.; Jeziorski, A.; Olszewski, W.; Senkus-Konefka, E.; Tchórzewska, H. Breast cancer. Onkol. Prak. Klin. 2015, 11 (Suppl. B), 24–52. Available online: file:///C:/Users/User/Downloads/42114-70242-1-SM%20(3).pdf (accessed on 13 March 2020).
- Sigl, V.; Penninger, J.M.R. RANK/RANKL-From bone physiology to breast cancer. Cytokine Growth Factor Rev. 2014, 25, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Husejko, J.; Porada, M.; Bieniek, D.; Skierkowska, N.; Prylińska, M.; Ruszkowska, N.; Nawrocka, A.; Sępka, J.; Szczesna, N.; Świerczek, P.; et al. Breast cancer as a significant social problem. J. Educ. Health Sport. 2019, 8, 412–423. [Google Scholar] [CrossRef]
- VanderWalde, A.; Hurria, A. Aging and osteoporosis in breast and prostate cancer. CA: A Cancer J. Clin. 2011, 61, 139–156. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.-C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genome Res. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.R.; Besser, D.; Kim, N.; Arron, J.; Vologodskaia, M.; Hanafusa, H.; Choi, Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 1999, 4, 1041–1049. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Israël, A. The IKK Complex, a Central Regulator of NF-κB Activation. Cold Spring Harb Perspect Biol. 2010, 3, a000158. [Google Scholar]
- Mizukami, J.; Takaesu, G.; Akatsuka, H.; Sakurai, H.; Ninomiya-Tsuji, J.; Matsumoto, K.; Sakurai, N. Receptor Activator of NF-κB Ligand (RANKL) Activates TAK1 Mitogen-Activated Protein Kinase Kinase Kinase through a Signaling Complex Containing RANK, TAB2, and TRAF6. Mol. Cell. Boil. 2002, 22, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Darnay, B.G.; Ni, J.; Moore, P.; Aggarwal, B.B. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 1999, 274, 7724–7731. [Google Scholar] [CrossRef] [Green Version]
- Takaesu, G.; Ninomiya-Tsuji, J.; Kishida, S.; Li, X.; Stark, G.R.; Matsumoto, K. Interleukin-1 (IL-1) Receptor-Associated Kinase Leads to Activation of TAK1 by Inducing TAB2 Translocation in the IL-1 Signaling Pathway. Mol. Cell. Boil. 2001, 21, 2475–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed] [Green Version]
- Chen, S.; Jin, G.; Huang, K.-M.; Ma, J.-J.; Wang, Q.; Ma, Y.; Tang, X.-Z.; Zhou, Z.-J.; Hu, Z.-J.; Wang, J.-Y.; et al. Lycorine suppresses RANKL-induced osteoclastogenesis in vitro and prevents ovariectomy-induced osteoporosis and titanium particle-induced osteolysis in vivo. Sci. Rep. 2015, 5, 12853. [Google Scholar] [CrossRef] [Green Version]
- Singhal, H.; Greene, M.E.; Zarnke, A.L.; Laine, M.; Al Abosy, R.; Chang, Y.-F.; Dembo, A.G.; Schoenfelt, K.; Vadhi, R.; Qiu, X.; et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget 2017, 9, 4282–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beleut, M.; Rajaram, R.D.; Caikovski, M.; Ayyanan, A.; Germano, D.; Choi, Y.; Schneider, P.; Brisken, C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA 2010, 107, 2989–2994. [Google Scholar] [CrossRef] [Green Version]
- Boonyaratanakornkit, V.; McGowan, E.; Sherman, L.; Mancini, M.A.; Cheskis, B.J.; Edwards, D.P. The Role of Extranuclear Signaling Actions of Progesterone Receptor in Mediating Progesterone Regulation of Gene Expression and the Cell Cycle. Mol. Endocrinol. 2007, 21, 359–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, E.; Skildum, A.; Piersonmullany, L.; Lange, C.A. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: Role of mitogen-activated protein kinases and cell cycle regulators. Steroids 2005, 70, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Duheron, V.; Hess, E.; Duval, M.; Decossas, M.; Castaneda, B.; Klöpper, J.E.; Amoasii, L.; Barbaroux, J.B.; Williams, I.R.; Yagita, H.; et al. Receptor activator of NF-kappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc. Natl. Acad. Sci. USA 2011, 13, 5342–5347. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.A. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J. Steroid Biochem. Mol. Boil. 2007, 108, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Stice, J.P.; Kazmin, D.; Wittmann, B.M.; Kimbrel, E.A.; Edwards, D.P.; Chang, C.Y.; McDonnell, D.P. Mechanisms of Progesterone Receptor Inhibition of Inflammatory Responses in Cellular Models of Breast Cancer. Mol. Endocrinol. 2010, 12, 2292–2302. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Valdivia, R.; Lydon, J.P. From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol. Cell. Endocrinol. 2011, 357, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, H.B.; Baker, R.; Owston, M.A.; Escalona, R.; Dick, E.J.; VandeBerg, J.L.; Nickisch, K.J. An efficient model of human endometriosis by induced unopposed estrogenicity in baboons. Oncotarget 2016, 7, 10857–10869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chwalisz, K.; Perez, M.C.; DeManno, D.; Winkel, C.; Schubert, G.; Elger, W. Selective Progesterone Receptor Modulator Development and Use in the Treatment of Leiomyomata and Endometriosis. Endocr. Rev. 2005, 26, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak-Świątkiewicz, K. Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression. Molecules 2019, 24, 3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Progestins | SPRMs | |||
|---|---|---|---|---|
| Progesterone | Promegestone | Mifepristone | Ulipristal | Asoprisnil |
 |  |  |  |  |
| Promegestone | Mifepristone | Asoprisnil | Ulipristal | |
|---|---|---|---|---|
| EC50 nM | 56.22 | 113 | 20.25 | 94.33 |
| Relative Potency [EC50 Test Compound/EC50 Promegestone] | 1 | 2.0 | 0.36 | 1.67 |
| Efficacy [% max. signal EC50 Promegestone] | NE | 23 | 82 | 27 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczak-Świątkiewicz, K. New Selective Progesterone Receptor Modulators and Their Impact on the RANK/RANKL Complex Activity. Molecules 2020, 25, 1321. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061321
Błaszczak-Świątkiewicz K. New Selective Progesterone Receptor Modulators and Their Impact on the RANK/RANKL Complex Activity. Molecules. 2020; 25(6):1321. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061321
Chicago/Turabian StyleBłaszczak-Świątkiewicz, Katarzyna. 2020. "New Selective Progesterone Receptor Modulators and Their Impact on the RANK/RANKL Complex Activity" Molecules 25, no. 6: 1321. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061321