Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. Metabolites Identification Using UHPLC-QTOF/MS
2.2. Reverse phase HPLC Method Development and Validation for RES and AJM Quantification
2.3. Anti-Amyloidogenic Effect of RES and AJM
2.4. Evaluation of Inhibition of β Sheet Formation
2.5. Morphological Validation of Inhibition of Aβ42 Aggregates Formation
2.6. RES and AJM Protects PC12 Cells Against Aβ42 Inflicted Cytotoxicity
2.7. In Vitro Neuroprotection Against Oxidative Stress
2.8. Evaluation of Dual Anti-Cholinesterase Potential of RES and AJM
2.9. β-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibition
2.10. Inhibition of Monoaminoxidase-B Enzyme
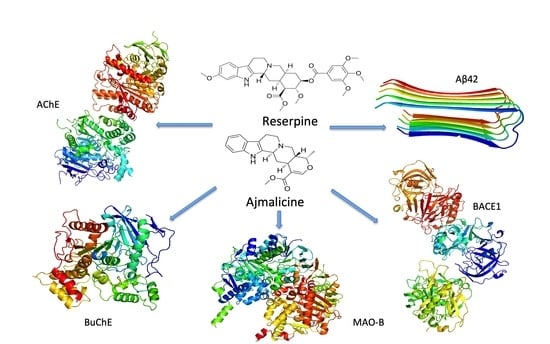
2.11. Molecular Interaction of RES and AJM with AD Targets
2.12. Pharmacokinetics Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydro-Alcoholic Extract
4.3. Liquid Chromatography-Mass Spectrometry
4.4. Reverse Phase-High Pressure Liquid Chromatography
4.5. Preparation of Aβ42 Fibrils
4.6. ThT Fluorescence Spectroscopy Measurements
4.7. Congo Red Binding Assay
4.8. Circular Dichroism Spectroscopy
4.9. Transmission Electron Microscopy
4.10. Aβ42 Induced Neurotoxicity in PC12 Cells
4.11. Neuroprotection Against Oxidative Stress Cytotoxicity Using H2O2
4.12. In Vitro ChE Enzyme Assay
4.13. In Vitro BACE-1 Enzyme Inhibition Assay
4.14. Monoaminoxidase-B (MAO-B) Inhibition Assay
4.15. Molecular Docking Analysis by Autodock 4.2
4.16. Pharmacokinetic Analysis (ADMET)
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Price, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. World Alzheimer Report 2016 Improving Healthcare for People Living with Dementia. Available online: http://eprints.lse.ac.uk/67858 (accessed on 19 December 2019).
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Hoozemans, J.J.; Van der Vies, S.M.; Rozemuller, A.J.; Van Horssen, J.; De Vries, H.E. Amyloid beta induces oxidative stress-mediated blood–brain barrier changes in capillary amyloid angiopathy. Antioxid. redox Signal. 2011, 15, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–186. [Google Scholar] [CrossRef]
- Hughes, R.E.; Nikolic, K.; Ramsay, R.R. One for all? Hitting multiple Alzheimer’s disease targets with one drug. Front. Neurosci. 2016, 10, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Melnikova, I. Therapies for Alzheimer’s disease. Nat. Rev. Drug Discov. 2007, 6, 341–342. [Google Scholar] [CrossRef]
- Pera, M.; Camps, P.; Munoz-Torrero, D.; Perez, B.; Badia, A.; Guillen, M.V. Undifferentiated and differentiated PC12 cells protected by huprines against injury induced by hydrogen peroxide. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Malagelada, C.; Greene, L.A. PC12 cells as a model for Parkinson’s disease research. In Parkinson’s Disease; Academic press: Cambridge, MA, USA, 2008; pp. 375–387. [Google Scholar]
- Rungsung, W.; Dutta, S.; Mondal, D.N.; Ratha, K.K.; Hazra, J. Pharmacognostical Profiling on the Root of Rauwolfia serpentina. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 612–616. [Google Scholar]
- Mittal, D.B.; Meenakshi, D.; Sharma, D.A.; Kumar, D.V. Phytochemical & pharmacological activity of rauwolfia serpentina–a review. Int. J. Ayurvedic Herb. Med. 2012, 2, 118–130. [Google Scholar]
- Luxenberg, J.; Feigenbaum, L.Z. The use of reserpine for elderly hypertensive patients. J. Am. Geriatr. Soc. 1983, 31, 556–559. [Google Scholar] [CrossRef]
- Roberts, M.F. Alkaloids: Biochemistry, ecology, and medicinal applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Guideline, I.H. Validation of Analytical Procedures: Text and Methodology Q2 (R1); International conference on harmonization: Geneva, Switzerland, 2005; pp. 11–12. [Google Scholar]
- Hoyer, W.; Grönwall, C.; Jonsson, A.; Ståhl, S.; Härd, T. Stabilization of a β-hairpin in monomeric Alzheimer’s amyloid-β peptide inhibits amyloid formation. Proc. Natl. Acad. Sci. USA 2008, 105, 5099–5104. [Google Scholar] [CrossRef] [Green Version]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberds, S.L.; Anderson, J.; Basi, G.; Bienkowski, M.J.; Branstetter, D.G.; Chen, K.S.; Freedman, S.; Frigon, N.L.; Games, D.; Hu, K.; et al. BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001, 10, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. New Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J. Alzheimer’s disease: The amyloid cascade hypothesis: An update and reappraisal. J. Alzheimer’s Dis. 2006, 9, 151–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, Y.P.; Or, T.C.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Sagi, S.; Avula, B.; Wang, Y.H.; Khan, I.A. Quantification and characterization of alkaloids from roots of Rauwolfia serpentina using ultra-high performance liquid chromatography-photo diode array-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 177–190. [Google Scholar] [CrossRef]
- Srivastava, A.; Tripathi, A.K.; Pandey, R.; Verma, R.K.; Gupta, M.M. Quantitative determination of reserpine, ajmaline, and ajmalicine in Rauvolfia serpentina by reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2006, 44, 557–560. [Google Scholar] [CrossRef]
- Du, Y.; Feng, M.; Wang, Y.N.; Cheng, L.; Wang, M.; Zhao, C.J. Simultaneous Determination of Reserpine, Yohimbine and Ajmalicine in the Extract of Rauvolfia Rootstocks, Branches and Leaves by HPLC. Asian J. Chem. 2014, 26, 7245–7248. [Google Scholar] [CrossRef]
- Urbanc, B.; Betnel, M.; Cruz, L.; Bitan, G.; Teplow, D.B. Elucidation of amyloid β-protein oligomerization mechanisms: Discrete molecular dynamics study. J. Am. Chem. Soc. 2010, 132, 4266–4280. [Google Scholar] [CrossRef] [Green Version]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Réfrégiers, M. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 3095–3103. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.S.; Xie, S.S.; Li, S.Y.; Pan, L.F.; Wang, X.B.; Kong, L.Y. Design, synthesis and evaluation of novel tacrine-(β-carboline) hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorganic Med. Chem. 2014, 22, 6089–6104. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.W.; Wang, J.; Zhu, Z.J.; Johnson, C.H.; Patti, G.J.; Siuzdak, G. Liquid chromatography quadrupole time-of-flight characterization of metabolites guided by the METLIN database. Nat. Protoc. 2013, 8, 451–460. [Google Scholar]
- Kashyap, P.; Muthusamy, K.; Niranjan, M.; Trikha, S.; Kumar, S. Sarsasapogenin: A steroidal saponin from Asparagus racemosus as multi target directed ligand in Alzheimer’s Disease. Steroids 2020, 153, 108529. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.H. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shafer, T.J.; Atchison, W.D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: A model for neurotoxicological studies. Neurotoxicology 1991, 12, 473–492. [Google Scholar] [PubMed]
- Kumar, S.; Seal, C.J.; Okello, E.J. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Withania somnifera roots. Int. J. Pharm. Sci. Res. 2011, 2, 1188–1192. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
Sample Availability: Samples of the compounds are available from Sigma Aldrich. |















| Peak No. | Compound Name | Formula | Monoisotopic Mass | Retention Time (min) | m + z Values |
|---|---|---|---|---|---|
| 1 | Behenic Acid | C22H44O2 | 340.592 | 5.7 | 341.578 |
| 2 | Sarpagine | C19H22N2O2 | 310.168 | 8.5 | 311.168 |
| 3 | Ajmaline | C20H26N2O2 | 326.199 | 12.9 | 327.199 |
| 4 | Ajmalicine | C21H24N2O3 | 352.179 | 13.4 | 353.177 |
| 5 | Yohimbine | C21H26N2O3 | 354.194 | 13.7 | 355.196 |
| 6 | Serpentine | C21H20N2O3 | 348.147 | 14.6 | 349.148 |
| 7 | Reserpine | C33H40N2O9 | 608.273 | 15.3 | 609.271 |
| 8 | Deserpedine | C32H38N2O8 | 578.263 | 15.9 | 579.263 |
| 9 | Reserpiline | C23H28N2O5 | 412.2 | 16.3 | 413.202 |
| 10 | Alpha or Beta Amyrin | C30H50O | 426.291 | 18.6 | 427.292 |
| 11 | Indobine | C11H11NO2 | 119.073 | 19.2 | 120.083 |
| 12 | Rutin | C27H30O16 | 611.52 | 20.6 | 612.521 |
| 8 | Unknown_RS1 | 15.9 | 290.264 | ||
| 9 | Unknown_RS2 | 16.3 | 653.239 | ||
| 11 | Unknown_RS3 | 19.2 | 381.288 | ||
| 13 | Unknown_RS4 | 20.6 | 685.422 |
| Indole Alkaloids | tR * (min) | Peak Asymmetry | RSD (%) | S/N LOD (ppm) | S/N LOQ (ppm) | Linear Regression Equation (Y = AX + C) |
|---|---|---|---|---|---|---|
| Ajmalicine | 6.1 ± 0.2 | 0.91 | 2.3 | 3.7 | 10.5 | y = 1.5808 x + 0.0495 R² = 0.99984 |
| Reserpine | 5.3 ± 0.1 | 0.94 | 1.8 | 2.82 | 9.76 | y = 130306 x − 5265.8 R² = 0.99998 |
| Secondary Structure | Aβ42 Control (%) | Aβ42 + RES (%) | Aβ42 + AJM (%) |
|---|---|---|---|
| α-helix | 0.0 | 0.0 | 0.0 |
| β-sheet (antiparallel) | 74.9 | 27.3 | 35.2 |
| β-sheet (parallel) | 16.5 | 0.0 | 0.0 |
| Turn | 8.6 | 17.8 | 11.7 |
| Others | 0.0 | 54.9 | 53.1 |
| Compound | IC50 (μM ± SD)/IC50 (µg/mL ± SD) | SI | |
|---|---|---|---|
| AChE IC50 | BuChE IC50 | ||
| Reserpine | 1.7 ± 2.08 µM | 2.8 ± 1.84 µM | 1.65 |
| Ajmalicine | 3.5 ± 1.41 µM | 5.44 ± 1.75 µM | 1.55 |
| R. serpentina extract | 14 ± 3.62 µg/ml | 22 ± 3.10 µg/ml | 1.57 |
| Donepzil | 0.98 ± 1.20 µM | 1 ± 2.89 µM | 1.02 |
| S.No. | Ligand | AD Target | Binding Energy (Kcal/mol) | No. of H-bonds | Interacting Residues | Bond Angle (Å) |
|---|---|---|---|---|---|---|
| 1. | RES (Reserpine) | Aβ42 | −9.45 | 5 | Asp23, Gly33, Lys28, Leu34, Val36 | 3.2, 2.4, 2.4, 3.3, 3.3, hydrophobic with Lys28 |
| AChE | −11.42 | 7 | Phe295, Arg296, Tyr337, Ser125, Glu334, Tyr72 | 2.3, 3.5, 1.9, (2.6, 2.3), 2.4, 2.7 | ||
| BuChE | −7.68 | 4 | Asn68, Thr120, Ala277, Val288 | 3.4, 2.3, 3.4, 2.4 | ||
| BACE-1 | −8.8 | 5 | Thr72, Asp32, Asp217, | 2.6, (3.2, 3.3), (3.5, 3.0) | ||
| MAO-B | −3.7 | 3 | Val85, Tyr326, Ile199 | 3.4, 2.4, 2.8 | ||
| 2. | AJM (Ajmalicine) | Aβ42 | −8.4 | 1 | Asp 23 | 3.2 |
| AChE | −9.7 | 3 | Phe295, Tyr286, Phe297 | 2.1, 3.0, 3.2 | ||
| BuChE | −6.6 | 4 | Asn68, Asp70, Trp82, Thr120 | 3.6, 2.1, 2.5, 2.1 | ||
| BACE-1 | −8.9 | 0 (all hydro-phobic) | Asp32, Asp228 | |||
| MAO-B | −5.6 | 6 | Glu85, Pro102, Thr202, Glu84 | 3.3, 2.4, 2.1, (3.2, 3.3) | ||
| Positive Control | ||||||
| 3. | Tannic acid | Aβ42 | −6.5 | 2 | Asp23, Lys28 | 2.1, 1.9 |
| 4. | Galanthamine | AChE | −10.8 | 3 | Tyr337, Glu202, Ser203 | 1.8, 2.2, 1.7 |
| 5. | Tacrine | BuChE | −6.52 | 1 | Trp82 | 2.3 |
| 6. | BXD | BACE-1 | −10.4 | 4 | ASP32, GLY34, PHE108, ASP217 | 1.9, 2.2, 2.5, 2.1 |
| 7. | Rasagilline | MAO-B | −7.5 | 0 (all hydro-phobic) | Gln206, Phe343, Tyr326, Leu171 | |
| Compound | MW (g/mol) | logP | AlogP | HBA | HBD | TPSA | nHB | nAcidic group | Filter L/B |
|---|---|---|---|---|---|---|---|---|---|
| Reserpine | 608.27 | 2.672 | −0.857 | 11 | 1 | 114.02 | 12 | 0 | B |
| Ajmalicine | 352.18 | 1.906 | −0.068 | 5 | 1 | 50.8 | 6 | 0 | L/B |
| Tacrine | 198.12 | 1.121 | −0.748 | 2 | 1 | 38.38 | 3 | 0 | L/B |
| Galanthamine | 287.15 | 1.197 | −0.444 | 4 | 1 | 41.93 | 5 | 0 | L/B |
| Donepezil | 379.21 | 2.633 | 0.364 | 4 | 0 | 38.77 | 4 | 0 | L/B |
| Rasagiline | 171.1 | 1.446 | 1.092 | 1 | 1 | 12.03 | 2 | 0 | L/B |
| Tannic acid | 1701.17 | 9.537 | −5.356 | 46 | 25 | 777.9 | 71 | 0 |
| Variables | Conditions |
|---|---|
| System | Agilent 6520 QTOF LC/MS system |
| Software | Agilent MassHunter B.05.00 |
| Column | Zorbax UPLC C18 (100 mm × 2.1 mm, i.d. 1.7 µm) |
| Mobile phase | Water with 0.01% formic acid (A) and methanol (B) |
| Flow Rate | 0.4 mL/min |
| Fragmentor voltage | 150 V |
| Electron spray ionization (ESI) mode | Positive |
| Mass-to-charge (m/z) scanning ratio | 100–1000 m/z |
| Injection volume | 3 µL |
| Column Temperature | 25 °C |
| Elution | Linear gradient: 0–5 min, 10–20% B 5–14 min, 20–30% B 14–18 min, 30–75% B 18–22 min, 75–100% B 22–31 min, 10 %B |
| Variables | Conditions for RES | Conditions for AJM |
|---|---|---|
| Absorbance maxima (λ) | 260 nm | 268 nm |
| Column | Zorbax HPLC C18 (150 mm × 4.6 mm, i.d. 5 µm) | Inertsil HPLC C8 (250 mm × 4.6 mm, i.d. 5µm) |
| Mobile phase | Water:Methanol (20:80 v/v) | Water:Acetonitrile (30:70 v/v) |
| Flow Rate | 1 mL/min | 0.5 mL/min |
| Injection volume | 10 µl | 20 µl |
| Elution | Isocratic | Isocratic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashyap, P.; Kalaiselvan, V.; Kumar, R.; Kumar, S. Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease. Molecules 2020, 25, 1609. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071609
Kashyap P, Kalaiselvan V, Kumar R, Kumar S. Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease. Molecules. 2020; 25(7):1609. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071609
Chicago/Turabian StyleKashyap, Priya, Vivekanandan Kalaiselvan, Robin Kumar, and Suresh Kumar. 2020. "Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease" Molecules 25, no. 7: 1609. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071609