1. Introduction
The demand for natural formulation active/inactive ingredients is increasing due to the increased risk of side effects posed by the synthetic compounds. Pharmaceutical ingredients of plant origin are generally safer as they produce less toxic metabolites. Various diseases (such as diabetes, cancer, stroke, Alzheimer’s and atherosclerosis), have been safely treated with natural entities and antioxidant-based formulations [
1]. Nowadays, bioactive natural oils play vital roles in the development of new drugs, especially for antitumor, antimicrobial, and psychoactive agents. The outstanding pharmacological benefits of these ingredients attract scientists to investigate more on the characterization of their biological profile. Such interesting ingredients increase the possibility of obtaining new targeted therapies for many challenging diseases [
2].
Curcumin (CUR,
Figure 1A), a highly lipophilic bioactive constituent extracted from the rhizomes of the herb
Curcuma longa L., has been reported to exhibit various biological and pharmacological effects (
Table 1). In animal models, oral administration of CUR inhibited cancer of lung [
3], skin [
4], neck and head [
5], oral [
6], hepatocellular carcinoma [
7]
, lymphomas, mammary tumors and leukemias [
8]. Because of its attractive properties, CUR is marketed in several countries worldwide and in different dosage forms. However, despite its promising pharmacological effects and safety, the clinical use of CUR has been limited by its poor bioavailability, which was correlated to its low aqueous solubility, extensive hepatic and intestinal metabolism, and fast systemic elimination [
9]. Even at a high dose, serum concentration of CUR was very low (only 1% in rat) [
10].
These limitations should find a solution through using novel drug delivery systems. Therefore, it is very important to increase CUR aqueous solubility and decrease its metabolic clearance simultaneously. An interesting approach for improving the delivery of CUR is co-administration with piperine (PP,
Figure 1B). PP is a major component of black pepper that exhibits several beneficial biological effects. PP acts as hepatic and intestinal glucuronidation inhibitor and has been reported to enhance the extent of absorption, serum concentration, and bioavailability of CUR in rats as well as humans [
11]. In particular, concomitant administration of PP along with CUR produced a 2000% increase in CUR bioavailability compared to CUR alone in humans. In addition, the combination of PP with CUR showed significant potentiation of its neurotransmitter enhancing (serotonin and dopamine), anti-immobility, and monoamine oxidase inhibitory effects as compared to the CUR effect alone [
12]. These studies provide a scientific rationale for the co-administration of PP with CUR to enhance the latter bioavailability and therapeutic efficacy.
Self-emulsifying, microemulsifying and nanoemulsifying drug delivery systems (SEDDS/SMEDDS/SNEDDS) are highly effective in enhancing the aqueous solubility, dissolution and bioavailability of poorly-water soluble drugs [
13,
14]. These lipid-based systems are composed of isotropic mixtures of oils, surfactant, cosurfactants and/or cosolvents. According to the “Lipid Formulation Classification System”, the oil proportion might range from 100% (Type I), 40–80% (Type II and IIIA), <20% (Type IIIB) or even 0% (Type IV) [
15,
16]. The utilized oil in these systems can be natural, synthetic or semi-synthetic. In the conventional SEDDS, oils are used as inactive ingredients (lipophilic solubilizers) to increase the loading of poorly-water soluble drugs. Several articles have shown the beneficial role of conventional SEDDS enhancing the aqueous solubility, absorption and bioavailability of CUR or PP single administration [
10,
17,
18,
19]. Furthermore, the combined CUR-PP SMEDDS improved CUR water solubility, stability and anti-colitis activity [
18]. However, none of these articles explored the potential of incorporating bioactive oils into CUR-PP SNEDDS formulation. Due to the outstanding health benefits of bioactive natural oils, it is worthy to investigate the feasibility of incorporating them in SNEDDS formulation. This strategy could lead to development of a new generation of novel bioactive lipid-based formulations called Bio-SNEDDS, which offer dual benefits; enhancing the dissolution and bioavailability of poorly-water soluble drugs along with delivering such beneficial bioactive oils to the patient.
In the current study, four bioactive oils namely, black seed oil (BSO), avocado oil (AVO), apricot oil (APO) and
Zanthoxylum rhetsa seed oil (ZRO) were investigated in the formulation of Bio-SNEDDS for CUR and PP delivery. In addition, these bioactive oils have several valuable nutritive and therapeutic effects on human health (
Table 1). Furthermore, to avoid lipid oxidation and improve the overall formulation stability, the optimized liquid SNEDDS were solidified using the two adsorbent grades Neusilin
® US2 and Aeroperl
® 300.
The proposed formula provides a novel strategy to develop Bio-SNEDDS formulations of CUR–PP using bioactive oil excipients. The co-delivery systems were characterized in terms of equilibrium solubility, appearance, droplet size, zeta potential and in-vitro dissolution.
3. Discussion
L-SNEDDS have demonstrated potential enhancement in the solubility of poorly water-soluble drugs and consequently improved drug bioavailability [
15]. However, L-SNEDDS suffer from several stability limitations such as rancidity, incompatibility with capsule shell, risk of formulation leakage, and the possibility of drug precipitation [
42,
43,
44,
45]. Furthermore, some drugs might undergo chemical degradation in the presence of SNEDDS components such as oils and related excipients [
45]. Solidification of L-SNEDDS possesses high potential to overcome such limitations along with retaining the solubilization benefits of L-SNEDDS.
Various CUR-PP loaded L-SNEDDS were prepared and the most potential candidates were solidified using the two adsorbents A300 and NUS. In order to obtain an optimum SNEDDS formula, six formulations were screened against their droplet size, zeta potential and equilibrium drug solubility parameters [
13]. CUR and PP are poorly-water soluble drugs with partition coefficient values; log
p ≈ 3 and 2.25, respectively [
17,
46]. The intermediate log
p values reveal the expected affinity of the two drugs towards cosolvents and polar oils, which was confirmed by the high CUR and PP solubility in (F6, LFCS Type IIIB systems) (
Table 2). However, PP showed high solubility also in Type II and Type IIIA systems that might be correlated to the presence of ZRO oil within these formulations. In particular, CUR implies higher solubility in the co-solvent Transcutol P (TcP), which reveals the fact that CUR has a hydrophobic moiety with less affinity towards Type I and Type II systems that contain significant amount of lipophilic materials [
15,
47].
Droplet size distribution is one of the significant parameters affecting the fate of emulsions in-vivo because it can influence the rate and extent of drug release [
48]. The smaller the globule size of the emulsion, the larger the surface area provided for drug absorption. The Z-average size of drug-free and drug-loaded F6-BSO:I988:TcP (2:2;1)/CrRH40 [1:1] was found to be 25 and 51 nm, respectively. The ultra-low droplet size of F6 was highly desirable and was attributed to one or more of the following reasons below:
- (a)
The high proportion of hydrophilic excipients in the formulation F6 (Type IIIB system) [
15].
- (b)
The inclusion of BSO, which possess good self-emulsification properties as confirmed by the significantly (
p < 0.05) lower droplet size of both formulations containing BSO (F3 and F6,
Figure 3). Similar low droplet size results were reported with other SNEDDS systems containing BSO [
46].
- (c)
The inclusion of the water soluble cosolvent TcP in the formulation.
- (d)
The inclusion of the highly hydrophilic surfactant Cr-RH40 that has higher HLB (14–16) compared to HCO-40 (12.5) and T85 (11).
Emulsion droplet charge is another parameter in evaluating emulsification efficiency [
49]. The significance of zeta potential value could be related to the stability of colloidal dispersions. Colloids with high zeta potential (negative or positive) are electrically stabilized and vice versa. In the current study, F5 showed the highest magnitude of zeta potential values, which gives an indication of system stability and could be attributed to the presence of non-ionic surfactants, adsorption of anionic species (such as hydroxyl ions from the water) to the droplet surfaces, or the existence of some anionic impurities in the surfactant (such as free fatty acids) [
50,
51] (
Figure 4).
SNEDDS (represented by F6) showed a maximum of 43% CUR precipitation in FaSSIF media, which was reduced to 20% CUR precipitation upon shifting to FeSSIF (
Figure 5). These data confirm that FeSSIF was able to minimize CUR precipitation, which is attributed to its higher contents of bile salt/phospholipids, thus playing a vital role in solubilizing hydrophobic and lipophilic molecules [
52]. On the other hand, the representative F6-SNEDDS showed less PP precipitation compared to CUR. Overall, a maximum of 6% PP was precipitated in both FaSSIF and FeSSIF media (
Figure 5). This could be attributed to the higher affinity of PP towards F6 SNEDDS components (Table 6).
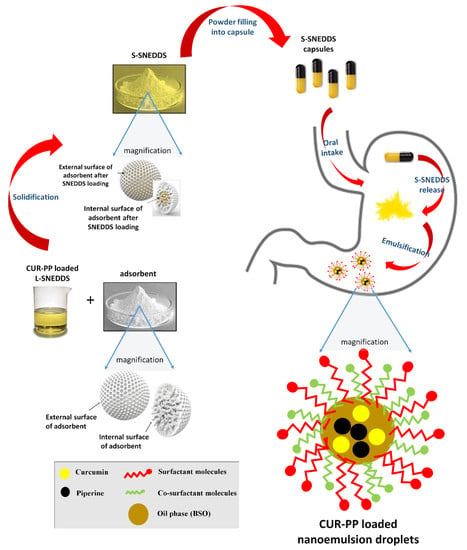
Solid SNEDDS powders were successfully obtained from liquid SNEDDS formulations by adsorption to solid carriers. The solid SNEDDS characterization revealed the presence of the CUR and PP in a molecularly dissolved amorphous state, within the SNEDDS formulation. These data confirm that both CUR and PP were completely solubilized within S-SNEDDS and that the solidification process did not trigger drug precipitation [
13,
41]. In addition, there was no obvious sign of chemical interaction between the drugs and the formulations.
The in-vitro dissolution studies revealed that both drugs are hydrophobic, in particular, CUR showed negligible drug dissolution at both SGF and SIF environments. These data emphasize the need for enhancing the oral CUR delivery by proper formulation design. Interestingly, CUR-PP solid SNEDDS showed superior (
p < 0.05) dissolution enhancement of both drugs at SGF and SIF. This was owing to the efficient self-nanoemulsification process that formed a favorable environment to maintain the drug solubilized, within the nano-sized oil droplets, upon exposure to GI fluids. In particular, F6 showed significantly higher CUR/PP dissolution efficiency compared to F1, F2 and pure drug powder (
Figure 10 and
Figure 12, and
Table 4). The superiority of F6 formulation could be attributed to one or more of the following reasons:
(1) F6 is LFCS Type IIIB system (enhanced self-emulsification efficiency) compared to F1 and F2, which are Type IIIA systems;
(2) The inclusion of BSO (F6) which possesses excellent self-emulsification properties compared to APO (F1) and AVO (F2) (
Figure 2 and
Figure 3);
(3) The inclusion of the cosolvent TcP in F6, which significantly increased the CUR and PP solubility within the SNEDDS;
(4) The significantly lower droplet size of F6 compared to F1 and F2.
(5) The inclusion of Cr-RH40 that possesses superior self-emulsification properties as revealed by a previous study that compared the performance of Cr-RH40 and HCO-40 by fixing the oil, cosurfactant and cosolvent and only varying the surfactant type within the formulation. The study revealed that Cr-RH40-based SNEDDS showed excellent (>80%) drug release and lower droplet size compared to HCO-40 counterparts [
46]. These findings were correlated with the higher HLB value of Cr-RH40 (HLB = 14–16) compared to HCO-40 (HLB = 12.5).
The increase in CUR release upon shifting to SIF could be owing to the weak acid property of CUR [
53]. In contrast, the release of the weak base PP was less affected than CUR by shifting into SIF. On a general basis, A300 presented lower CUR and PP release compared to NUS. This finding could be strongly correlated to the pore size distribution of each adsorbent. A300 is predominately mesoporous (2–50 nm pore size) with the majority of pores ranging from ≈10–30nm (Table 6) [
54]. In the current study, the three optimized SNEDDS formulations produce globules with diameters 51–263 nm which are larger than the diameter of the majority of A300 pores. Therefore, it is difficult for such systems to undergo complete emulsification inside the predominant mesoporous region, which hinders complete drug release and solubilization [
55]. On the other hand, NUS is predominantly macroporous (>50 nm pore size) with the majority of pores ranging from ≈700–950 nm (Table 6). Moreover, previous SEM images of plain NUS suggested a kind of surface porosity [
54]. The significantly larger pores provide the required room to undergo complete emulsification and hence enhanced drug solubilization. It is worth mentioning that the negative influence of A300 was more pronounced (
p < 0.05) in CUR compared to PP. The significant difference between CUR and PP release from the same adsorbent could be attributed to the strong physical bonds developed between A300 and CUR, which hindered drug release upon exposure to GI fluids. Similar results were observed with the adsorbents Syloid and NUS, and could be correlated with one or more the following factors: smaller pore size, longer pore channels and/or developed hydrogen bonding between the drug and adsorbent [
46,
54,
55,
56].
In overall assessment, F6N was selected as the optimal CUR-PP SNEDDS formulation due to the high CUR/PP solubility, lower droplet size, good dispersion results (of its liquid form) along with superior CUR-PP release from solid SNEDDS. Most importantly, F6N comprised the bioactive oil BSO. In several Islamic and Arabic countries, black seed is considered as one of the greatest forms of herbal healing medicine. Black seed contains over 100 phytochemical constituents which work together to produce a synergetic effect supporting the immune system and strengthening the body’s constitution. A recent review revealed that black seed (along with its oil) is a multi-disciplinary remedy that can successfully treat over 129 different types of human ailments [
33,
34]. In particular, black seed oil has a rich composition of several valuable components that play a vital part in forming prostaglandin (PG) E1, which balances and strengthens the immune system against infections, allergies and chronic illnesses [
33]. Many therapeutic properties of this plant were suggested to be due to the presence of thymoquinone as a major bioactive component of the essential oil [
34]. In addition, black seed oil contains antioxidants that protect the body from free radicals. BSO is also a tremendous source of essential fatty acids [
33]. Accordingly, F6N offers a potential oral dosage form for combined oral delivery of CUR-PP along with the bioactive BSO.