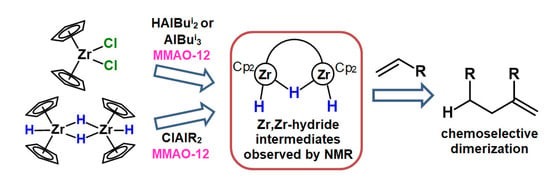
Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization
Abstract
:1. Introduction
2. Results
2.1. Study of 1-Alkene Transformations in Catalytic Systems Cp2ZrY2 (Y = Cl, H)-OAC-MMAO-12
2.1.1. Activity and Chemoselectivity of System Cp2ZrCl2-(AlR3 or HAlBui2)-MMAO-12 with Respect to Alkenes
2.1.2. Activity and Chemoselectivity of System [Cp2ZrH2]2-ClAlR2 (R = Me, Et, Bui)-MMAO-12 with Respect to Alkenes
2.2. NMR Study of Hydride Intermediate Structures in Systems Cp2ZrY2 (Y = Cl, H)-OAC-MMAO-12
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Reaction of Cp2ZrCl2 with OAC, MMAO-12 and 1-Alkene
4.3. Reaction of [Cp2ZrH2]2 with ClAlR2, MMAO-12 and 1-Alkene
4.4. NMR Study of the Reaction of Cp2ZrCl2 with XAlBui2 (X = H, Bui) and MMAO-12
4.5. NMR Study of the Reaction of [Cp2ZrH2]2 with ClAlR2 (R=Me, Et, Bui) and MMAO-12
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef]
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in a-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Carr, D.B.; Schwartz, J. Preparation of organoaluminum compounds by hydrozirconation-transmetalation. J. Am. Chem. Soc. 1979, 101, 3521–3531. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G. Hydrometallation of Unsaturated Compounds. In Modern Reduction Methods; Andersson, P.G., Munslow, I.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 447–489. [Google Scholar]
- Song, Z.; Takahashi, T. 8.23 Hydrozirconation of Alkenes and Alkynes. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherland, 2014; pp. 838–876. [Google Scholar]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4,658,078, 14 April 1987. [Google Scholar]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1)—A catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P.; Krüger, R.-P.; Hanselmann, R. Analyses of Propene and 1-Hexene Oligomers from Zirconocene/MAO Catalysts-Mechanistic Implications by NMR, SEC, and MALDI-TOF MS. Macromol. Chem. Phys. 2002, 203, 129–138. [Google Scholar] [CrossRef]
- Janiak, C.; Blank, F. Metallocene Catalysts for Olefin Oligomerization. Macromol. Symp. 2006, 236, 14–22. [Google Scholar] [CrossRef]
- Landis, C.R.; Christianson, M.D. Metallocene-catalyzed alkene polymerization and the observation of Zr-allyls. Proc. Natl. Acad. Sci. USA 2006, 103, 15349–15354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, M.E.; Dutta, S.; Veige, A.S. β-Alkyl Elimination: Fundamental Principles and Some Applications. Chem. Rev. 2016, 116, 8105–8145. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.; Cioni, P.; Wei, J. Asymmetric hydrooligomerization of propylene. J. Am. Chem. Soc. 1987, 109, 6189–6191. [Google Scholar] [CrossRef]
- Yu, Y.; Busico, V.; Budzelaar, P.H.M.; Vittoria, A.; Cipullo, R. Of Poisons and Antidotes in Polypropylene Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8590–8594. [Google Scholar] [CrossRef]
- Desert, X.; Proutiere, F.; Welle, A.; Den Dauw, K.; Vantomme, A.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Kirillov, E. Zirconocene-Catalyzed Polymerization of α-Olefins: When Intrinsic Higher Activity Is Flawed by Rapid Deactivation. Organometallics 2019, 38, 2664–2673. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The catalytic behavior of heterocenes activated by TIBA and MMAO under a low Al/Zr ratios in 1-octene polymerization. Appl. Catal. A-Gen. 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Soga, K.; Kaminaka, M. Polymerization of propene with the heterogeneous catalyst system Et[IndH4]2ZrCl2/MAO/SiO2 combined with trialkylaluminium. Makromol. Chem. Rapid Commun. 1992, 13, 221–224. [Google Scholar] [CrossRef]
- Resconi, L.; Piemontesi, F.; Nifant’ev, I.E.; Ivchenko, P.V. Metallocene Compounds, Process for Their Preparation, and Their Use in Catalysts for the Polymerization of Olefins. U.S. Patent 6,051,728, 18 April 2000. [Google Scholar]
- Bravaya, N.M.; Khrushch, N.E.; Babkina, O.N.; Panin, A.N. Formation and catalytic properties of metallocene systems with combined cocatalyst of Al(i-Bu)3 perfluorophenyl borate. Ross. Khimicheskij Zhurnal 2001, 45, 56–68. [Google Scholar]
- Sacco, M.; Nifant’ev, I.; Ivchenko, P.; Bagrov, V.; Focante, F. Metallocene Compounds. U.S. Patent 7,803,887 B2, 28 September 2010. [Google Scholar]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A-Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Becke, S.; Rosenthal, U. Aluminoxane Free Catalyst System, Useful for Polymerization of Alpha-Olefins, Comprises Fluorine Containing Metal Complex and Trialkyl or Triaryl Boron or Aluminum Compound. Patent DE 19,932,409A, 18 January 2001. [Google Scholar]
- Becke, S.; Rosenthal, U. Composition Based on Fluorine-Containing Metal Complexes. U.S. Patent 6,303,718 B1, 16 October 2001. [Google Scholar]
- Becke, S.; Rosenthal, U.; Baumann, W.; Arndt, P.; Spannenberg, A. Metallocyclocumulene Compounds Useful as Polymerization Catalysts Are New. Patent DE 10,110,227A1, 5 September 2002. [Google Scholar]
- Shoer, L.I.; Gell, K.I.; Schwartz, J. Mixed-metal hydride complexes containing Zr-H-Al bridges. synthesis and relation to transition-metal-catalyzed reactions of aluminum hydrides. J. Organomet. Chem. 1977, 136, c19–c22. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-catalyzed olefin hydroalumination by alkylalanes. Russ. Chem. Bull. 2005, 54, 316–327. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2·ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al Hydride Intermediate Structure and Dynamics in Alkene Hydroalumination with XAlBui2 (X = H, Cl, Bui), Catalyzed by Zr η5-Complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-Complexed Zirconocene Hydrides: Identification of Hydride-Bridged Species by NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.M.; Bercaw, J.E.; Henling, L.M.; Day, M.W.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides: NMR-Spectroscopic Identification, Crystallographic Structure Determination, and Interconversion with Other Zirconocene Cations. J. Am. Chem. Soc. 2011, 133, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Kovyazin, P.V.; Abdullin, I.g.N.; Parfenova, L.V. Diastereoselective synthesis of functionally substituted alkene dimers and oligomers, catalysed by chiral zirconocenes. Catal. Commun. 2019, 119, 144–152. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Metallocene Polymerization Catalysts. Synthesis and Properities of the First Base-Free Zirconocene Hydride. Angew. Chem. Int. Ed. 1992, 31, 1375–1377. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Zirconocene Olefin Polymerization Catalysts Based on the Organo-Lewis Acid Tris(pentafluorophenyl)borane. A Synthetic, Structural, Solution Dynamic, and Polymerization Catalytic Study. J. Am. Chem. Soc. 1994, 116, 10015–10031. [Google Scholar] [CrossRef]
- González-Hernández, R.; Chai, J.; Charles, R.; Pérez-Camacho, O.; Kniajanski, S.; Collins, S. Catalytic System for Homogeneous Ethylene Polymerization Based on Aluminohydride-Zirconocene Complexes. Organometallics 2006, 25, 5366–5373. [Google Scholar] [CrossRef]
- Arndt, P.; Baumann, W.; Spannenberg, A.; Rosenthal, U.; Burlakov, V.V.; Shur, V.B. Reactions of Titanium and Zirconium Derivatives of Bis(trimethylsilyl)acetylene with Tris(pentafluorophenyl)borane: A Titanium(III) Complex of an Alkynylboranate. Angew. Chem. Int. Ed. 2003, 42, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Arndt, P.; Jäger-Fiedler, U.; Klahn, M.; Baumann, W.; Spannenberg, A.; Burlakov, V.V.; Rosenthal, U. Formation of Zirconocene Fluoro Complexes: No Deactivation in the Polymerization of Olefins by the Contact-Ion-Pair Catalysts [Cp’2ZrR]+[RB(C6F5)3]−. Angew. Chem. Int. Ed. 2006, 45, 4195–4198. [Google Scholar] [CrossRef]
- Carr, A.G.; Dawson, D.M.; Thornton-Pett, M.; Bochmann, M. Cationic Zirconocene Hydrides: A New Type of Highly Effective Initiators for Carbocationic Polymerizations. Organometallics 1999, 18, 2933–2935. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [Green Version]
- Hölscher, M.; Keul, H.; Höcker, H. Evaluation of the Potential of [{Me2C(Cp)2}Zr(Me)]+ and [{Me2C(Cp)2}Zr(H)]+ as Single-Site Catalysts for Controlled Methyl Vinyl Ether Polymerizations by Density Functional Calculations. Organometallics 2003, 22, 1055–1064. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr,Al- hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Yoshida, T. A novel zirconium-catalyzed hydroalumination of olefins. Tetrahedron Lett. 1980, 21, 1501–1504. [Google Scholar] [CrossRef]
- Claridge, T.D.W. Chapter 8—Correlations through space: The nuclear Overhauser effect. In Tetrahedron Organic Chemistry Series; Claridge, T.D.W., Ed.; Elsevier: Amsterdam, The Netherland, 2009; Volume 27, pp. 247–302. [Google Scholar]
- Hassinen, A.; Martins, J.C.; Hens, Z. Solution NMR Toolbox for Colloidal Nanoparticles. In Nanoparticles: Workhorses of Nanoscience; de Mello Donegá, C., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; pp. 273–293. [Google Scholar]
- Ghiotto, F.; Pateraki, C.; Tanskanen, J.; Severn, J.R.; Luehmann, N.; Kusmin, A.; Stellbrink, J.; Linnolahti, M.; Bochmann, M. Probing the Structure of Methylalumoxane (MAO) by a Combined Chemical, Spectroscopic, Neutron Scattering, and Computational Approach. Organometallics 2013, 32, 3354–3362. [Google Scholar] [CrossRef]
- Zijlstra, H.S.; Joshi, A.; Linnolahti, M.; Collins, S.; McIndoe, J.S. Modifying methylalumoxane via alkyl exchange. Dalton Trans. 2018, 47, 17291–17298. [Google Scholar] [CrossRef] [Green Version]
- Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Macchioni, A.; Busico, V.; Ehm, C. On the Nature of the Lewis Acidic Sites in “TMA-Free” Phenol-Modified Methylaluminoxane. Eur. J. Inorg. Chem. 2020, 2020, 1088–1095. [Google Scholar] [CrossRef]
- Talsi, E.P.; Semikolenova, N.V.; Panchenko, V.N.; Sobolev, A.P.; Babushkin, D.E.; Shubin, A.A.; Zakharov, V.A. The metallocene/methylaluminoxane catalysts formation: EPR spin probe study of Lewis acidic sites of methylaluminoxane. J. Mol. Catal. A Chem. 1999, 139, 131–137. [Google Scholar] [CrossRef]
- Boccia, A.C.; Costabile, C.; Pragliola, S.; Longo, P. Selective Dimerization of γ-Branched α-Olefins in the Presence of C2v Group-4 Metallocene-Based Catalysts. Macromol. Chem. Phys. 2004, 205, 1320–1326. [Google Scholar] [CrossRef]
- Gunasekara, T.; Preston, A.Z.; Zeng, M.; Abu-Omar, M.M. Highly Regioselective α-Olefin Dimerization Using Zirconium and Hafnium Amine Bis(phenolate) Complexes. Organometallics 2017, 36, 2934–2939. [Google Scholar] [CrossRef]
- Small, B.L.; Marcucci, A.J. Iron Catalysts for the Head-to-Head Dimerization of α-Olefins and Mechanistic Implications for the Production of Linear α-Olefins. Organometallics 2001, 20, 5738–5744. [Google Scholar] [CrossRef]
- Small, B.L. Tridentate Cobalt Catalysts for Linear Dimerization and Isomerization of α-Olefins. Organometallics 2003, 22, 3178–3183. [Google Scholar] [CrossRef]
- Broene, R.D.; Brookhart, M.; Lamanna, W.M.; Volpe, A.F. Cobalt-Catalyzed Dimerization of α-Olefins to Give Linear α-Olefin Products. J. Am. Chem. Soc. 2005, 127, 17194–17195. [Google Scholar] [CrossRef]
- Hanton, M.J.; Daubney, L.; Lebl, T.; Polas, S.; Smith, D.M.; Willemse, A. Selective dimerisation of α-olefins using tungsten-based initiators. Dalton Trans. 2010, 39, 7025–7037. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Troyanov, S.I.; Meetsma, A.; Hessen, B.; Teuben, J.H. Regioselective Homo- and Codimerization of α-Olefins Catalyzed by Bis(2,4,7-trimethylindenyl)yttrium Hydride. Organometallics 1998, 17, 284–286. [Google Scholar] [CrossRef]
- Lee, D.W.; Yi, C.S. Chain-Selective and Regioselective Ethylene and Styrene Dimerization Reactions Catalyzed by a Well-Defined Cationic Ruthenium Hydride Complex: New Insights on the Styrene Dimerization Mechanism. Organometallics 2010, 29, 3413–3417. [Google Scholar] [CrossRef] [Green Version]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khursan, S.L.; Khalilov, L.M.; Dzhemilev, U.M. DFT and Ab Initio Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. II. Olefin Interaction with Catalytically Active Centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Schwartz, D.J.; Metz, M.V.; Marks, T.J.; Liable-Sands, L.; Rheingold, A.L. Coordination Copolymerization of Severely Encumbered Isoalkenes with Ethylene: Enhanced Enchainment Mediated by Binuclear Catalysts and Cocatalysts. J. Am. Chem. Soc. 2005, 127, 14756–14768. [Google Scholar] [CrossRef]
- Li, H.; Marks, T.J. Nuclearity and cooperativity effects in binuclear catalysts and cocatalysts for olefin polymerization. Proc. Natl. Acad. Sci. USA 2006, 103, 15295. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Mouat, A.R.; Motta, A.; Macchioni, A.; Zuccaccia, C.; Delferro, M.; Marks, T.J. Pyridylamido Bi-Hafnium Olefin Polymerization Catalysis: Conformationally Supported Hf···Hf Enchainment Cooperativity. ACS Catal. 2015, 5, 5272–5282. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Brainina, E.M.; Nesmeyanov, A.N. The synthesis of mixed pincerlike cyclopentadienyl compounds of zirconium. Dokl. Acad. Nauk SSSR 1961, 138, 1369–1372. [Google Scholar]
- Parkhurst, R.M.; Rodin, J.O.; Silverstein, R.M. Isolation, Identification, and Synthesis of Components of a “Styrene Dimer Fraction”. J. Org. Chem. 1963, 28, 120–123.s. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Entry | 1-Alkene | OAC | [Zr]:[Al]:[MAO]:[1-alkene] | T, °C | Time, min | Alkene Conversion, % | Product yield, 1 % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-D | 3-D | 4 | 5-D | 6 | |||||||
| 1 | 1-octene (1b) | HAlBui2 [32] | 1:60:0:50 | 20 | 180 | 11 | 11 | - | - | - | - |
| 2 | AlBui3 [32] | 83 | 83 | - | - | - | - | ||||
| 3 | AlBui3 | 1:60:30:50 | 20 | 180 | 98 | 91 | 2 | 5 | - | - | |
| 4 | HAlBui2 | 1:60:240:50 | 20 | 180 | 20 | 20 | - | - | - | - | |
| 5 | AlBui3 | 94 | 51 | 3 | 40 | - | - | ||||
| 6 | AlBui3 | 1:3:30:50 | 20 | 20 | 99 | 1 | 1 | 97 | - | - | |
| 7 | 1:3:30:100 | 20 | 30 | 99 | - | <1 | 98 | - | <1 (6′) 8 | ||
| 8 | HAlBui2 | 1:3:30:100 | 40 | 10 | 99 | - | 3 | 96 | - | - | |
| 9 | AlBui3 | 99 | - | 1 | 98 | - | - | ||||
| 10 | 1-hexene (1a) | HAlBui2 | 1:3:30:100 | 40 | 15 | 99 | 5 | 1 | 91 | - | 2 (6′) 8 |
| 11 | AlBui3 | 99 | 4 | 1 | 89 | - | 3 (6′) 8 | ||||
| 12 | AlMe3 | 91 | - | - | 87 | 2 (5′) | 2 (6′) | ||||
| 13 | AlEt3 | 92 | 2 | 3 | 68 | - | 6 (6′) 8 12 (6″) | ||||
| 14 | HAlBui2 | 1:3:30:250 | 60 | 5 | 98 | 1 | 1 | 94 | - | 2 (6′) 8 | |
| 15 | AlBui3 | 99 2 | 1 | 1 | 88 | - | 4 (6′) 8 | ||||
| 16 | AlMe3 | 99 3 | 1 | 1 | 85 | - | 3 (6′) | ||||
| 17 | AlEt3 | 96 3 | 1 | 1 | 85 | - | 1 (6′) 8 | ||||
| 18 | HAlBui2 | 1:3:30:500 | 60 | 5 | 98 | - | - | 97 | - | - | |
| 19 | AlBui3 | 98 | 1 | 1 | 93 | - | 1 (6′) 8 | ||||
| 20 | AlMe3 | 99 4 | 1 | 1 | 87 | - | 5 (6′) | ||||
| 21 | AlEt3 | 98 | 1 | 1 | 96 | - | <1 (6′) 8 | ||||
| 22 | HAlBui2 | 1:3:30:1000 | 60 | 15 | 99 | - | - | 98 | - | - | |
| 23 | 30 | 83 5 | - | - | 78 | - | - | ||||
| 24 | 60 | 86 5,6 | <1 | - | 83 | - | <1 (6′) 8 | ||||
| 25 | AlBui3 | 15 | 94 6 | - | - | 92 | - | 1 (6′) 8 | |||
| 26 | AlMe3 | 10 | 92 | 2 | - | 84 | - | 6 (6′) | |||
| 27 | AlEt3 | 60 | 65 7 | - | - | 64 | - | <1 (6″) | |||
| Entry | 1-Alkene | ClAlR2 | [Cp2ZrH2]: [Al]: [MAO]:[1-alkene] | T, °C | Time, min | Alkene Conversion, % | Product Yield, 1 % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-D | 3-D | 4 | 5-D | 6 | |||||||
| 1 | 1-hexene (1a) | ClAlMe2 | 1:3:30:100 | 20 | 60 | 32 | 2 | 7 | 15 | 1 (5′) | 7 (6′) |
| 2 | 180 | 99 | 2 | 6 | 80 | 2 (5′) | 9 (6′) | ||||
| 3 | ClAlEt2 | 60 | 75 | 2 | 4 | 47 | - | 22 (6″) | |||
| 4 | 180 | 99 | 2 | 5 | 69 | - | 23 (6″) | ||||
| 5 | ClAlBui2 | 60 | 79 | 1 | 5 | 73 | - | - | |||
| 6 | 180 | 99 | 1 | 2 | 97 | - | - | ||||
| 7 | 1-octene (1b) | ClAlMe2 | 60 | 25 2 | 1 | 4 | 15 | - | 4 (6′) | ||
| 8 | 180 | >99 2 | 4 | 10 | 72 | 3 | 9 (6′) | ||||
| 9 | ClAlEt2 | 60 | 73 2 | 2 | 13 | 46 | - | 11 (6′) 4 | |||
| 10 | 180 | >99 2 | 3 | 9 | 76 | 2 (5′) 4 | 9 (6′) 4 | ||||
| 11 | ClAlBui2 | 60 | 85 | 2 | 12 | 60 | - | 10 (6′) 4 | |||
| 12 | 180 | >99 2 | 3 | 9 | 77 | 2 (5′) 4 | 8 (6′) 4 | ||||
| 13 | 1-decene (1c) | ClAlMe2 | 60 | <5 | - | 2 | 3 | - | - | ||
| 14 | 180 | >99 | 3 | 6 | 81 | 1 (5′) | 8 (6′) | ||||
| 15 | ClAlEt2 | 60 | 68 | 4 | 10 | 28 | 5 (5″) | 21 (6″) | |||
| 16 | 180 | >99 | 6 | 4 | 76 | 2 (5′) 4 | 12 (6′) 4 | ||||
| 17 | ClAlBui2 | 60 | 73 | 4 | 7 | 59 | - | 2 (6′) 4 | |||
| 18 | 180 | >99 | 4 | 7 | 87 | - | 2 (6′) 4 | ||||
| 19 | 1-hexene (1a) | ClAlMe2 | 40 | 15 | 96 | 4 | 3 | 82 | - | 10 (6′) | |
| 20 | ClAlEt2 | 15 | 98 | 2 | 3 | 83 | - | 11 (6′) 4 | |||
| 21 | ClAlBui2 | 15 | >99 | 4 | 2 | 86 | - | 9 (6′) 4 | |||
| 22 | 1-octene (1b) | ClAlMe2 | 15 | 83 | 4 | 3 | 73 | - | 4 (6′) | ||
| 23 | ClAlEt2 | 15 | 89 | 4 | 3 | 79 | - | 1 (6′) 4 2 (6″) | |||
| 24 | ClAlBui2 | 15 | 98 | 5 | - | 91 | - | 2 (6′) 4 | |||
| 25 | 4-methyl-1-pente-ne (1d) | ClAlBui2 | 30 | >99 | 1 | 1 | 95 | - | 2 (6′) 4 | ||
| 26 | 1-hexene (1a) | ClAlMe2 | 60 | 5 | 99 | 1 | 1 | 91 | - | 6 (6′) | |
| 27 | ClAlEt2 | 5 | 99 | 1 | 2 | 92 | - | 5 (6′) 4 | |||
| 28 | ClAlBui2 | 5 | 99 | - | 2 | 94 | - | 3 (6′) 4 | |||
| 29 | ClAlMe2 | 1:3:30:250 | 60 | 5 | 94 | 1 | 1 | 85 | - | 6 (6′) | |
| 30 | ClAlEt2 | 5 | 98 3 | 1 | 2 | 90 | - | 5 (6″) | |||
| 31 | ClAlBui2 | 5 | 98 | 1 | 2 | 87 | - | 3 (6′) 4 | |||
| 32 | ClAlBui2 | 1:3:30:500 | 60 | 30 | 95 | 1 | 2 | 87 | - | 5 (6′) 4 | |
| 33 | ClAlBui2 | 1:3:30:1000 | 60 | 360 | 65 2 | 1 | 2 | 57 | - | 3 (6′) 4 | |
| 34 | ClAlBui2 | 1:3:3:100 | 20 | 240 | 41 | 2 | 3 | 35 | - | - | |
| 35 | ClAlBui2 | 1:3:12:100 | 20 | 240 | 99 | - | 13 | 86 | - | - | |
| 36 | ClAlBui2 | 1:3:60:100 | 20 | 15 | >99 | 2 | <1 | 81 | 2 (5′) 4 | 14 (6′) 4 | |
| 37 | ClAlBui2 | 1:3:120:100 | 20 | 15 | >99 | 1 | 2 | 80 | 1 (5′) 4 | 15 (6′) 4 | |
| 38 | Allylbenzene (1e) | ClAlBui2 | 1:3:30:100 | 100 | 60 | 94 | 6 | 2 | 52 + 9 5 | - | 25 (6′) 4 |
| 39 | Styrene (1f) | ClAlBui2 | 60 | 79 3 | 8 | - | 32 + 26 5 | - | 6 (6′) 4 | ||
| Complex | δH Cp | δC Cp | δH Zr-H-Zr | δH Zr-H-Al | δH MAO |
|---|---|---|---|---|---|
| 10a | 5.68 (s, 10H) | 104.74 | −2.43 (d, 8.6 Hz, 2H) −1.19 (t, 8.6 Hz, 1H) | ||
| 11a | 5.72 (s, 10H) | 107.90 | −2.55 (br.s, 1H) −1.60 (br.s, 1H) | ||
| 12а | 5.52 (s, 20H) | 108.00 | −6.64 (t, 17.6 Hz, 1H) −1.19 (d, 17.6 Hz, 2H) | ||
| 13a | 5.75 (br.s, 10H) | 108.29 (br.) | −0.60 (br.s, 2H) | ||
| 12a∙MAO | 5.42 | 107.88 | −6.56 (t, 17.2 Hz, 1Н) −1.08 (d, 17.2 Hz, 2Н) −6.71 (t, 17.6 Hz, 1Н) −1.27 (d, 17.6 Hz, 2Н) | ||
| 12a∙MAO (heavy phase) | 5.11–5.33 | 107.59 (br.) | −6.92 (br.t, 2H) −1.44 (br.d, 2H) | −0.63 ÷ −0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092216
Parfenova LV, Kovyazin PV, Bikmeeva AK. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules. 2020; 25(9):2216. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092216
Chicago/Turabian StyleParfenova, Lyudmila V., Pavel V. Kovyazin, and Almira Kh. Bikmeeva. 2020. "Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization" Molecules 25, no. 9: 2216. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092216