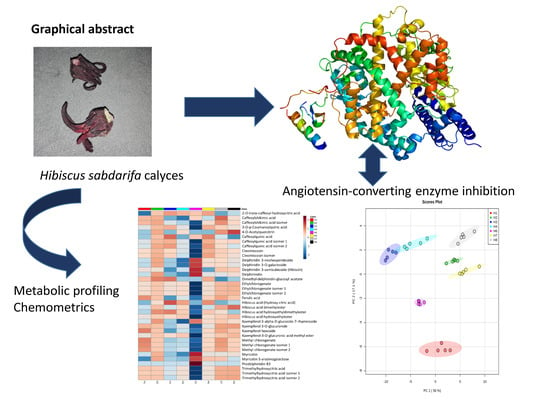
Optimization of an Extraction Solvent for Angiotensin-Converting Enzyme Inhibitors from Hibiscus sabdariffa L. Based on Its UPLC-MS/MS Metabolic Profiling
Abstract
:1. Introduction
2. Results
2.1. ACE Inhibition Assay
2.2. UPLC-MS/MS Analysis and Metabolite Identification
2.3. Effect of Extraction Solvent on Metabolite Abundance
2.4. Metabolites with the Highest ACE Inhibition Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Quantitative Estimation of the Total Phenolic Content
4.4. ACE Inhibition Assay
4.5. UPLC-MS/MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajay, M.; Chai, H.J.; Mustafa, A.M.; Gilani, A.H.; Mustafa, M.R. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J. Ethnopharmacol. 2007, 109, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, Y.W.; Chuah, L.O.; Ahmad, R.; Bhat, R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. J. King Saud Univ. Sci. 2013, 25, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus Sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukwuani-Kwaja, A. Antihypertensive activity of Hibiscus Sabdariffa aqueous calyx extract in Albino rats. Sky J. Biochem. Res. 2015, 4, 13–15. [Google Scholar]
- Nwachukwu, D.; Aneke, E.; Nwachukwu, N.; Obika, L.; Nwagha, U.; Eze, A. Effect of Hibiscus sabdariffa on blood pressure and electrolyte profile of mild to moderate hypertensive Nigerians: A comparative study with hydrochlorothiazide. Niger. J. Clin. Pract. 2015, 18, 762–770. [Google Scholar] [CrossRef]
- Hajifaraji, M.; Haji Tarkhani, A.H. The effect of sour tea (Hibiscus Sabdariffa) on essential hypertension. J. Ethnopharm. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Alarcón-Alonso, J.; Zamilpa, A.; Alarcon, F.; Lucila, M.; Tortoriello, J.; Jimenez-Ferrer, J. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J. Ethnopharmacol. 2012, 139, 751–756. [Google Scholar] [CrossRef]
- Nwachukwu, D.C.; Aneke, E.I.; Obika, L.F.; Nwachukwu, N.Z. Effects of aqueous extract of Hibiscus sabdariffa on the renin-angiotensin-aldosterone system of Nigerians with mild to moderate essential hypertension: A comparative study with lisinopril. Indian J. Pharmacol. 2015, 47, 540–545. [Google Scholar] [CrossRef]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Salem, M.A.; De Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the Context of Plant Natural Products Research: From Sample Preparation to Metabolite Analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Vargas-León, E.A.; Díaz-Batalla, L.; González-Cruz, L.; Bernardino-Nicanor, A.; Castro-Rosas, J.; Reynoso-Camacho, R.; Gómez-Aldapa, C.A. Effects of acid hydrolysis on the free radical scavenging capacity and inhibitory activity of the angiotensin converting enzyme of phenolic compounds of two varieties of jamaica (Hibiscus sabdariffa). Ind. Crop. Prod. 2018, 116, 201–208. [Google Scholar] [CrossRef]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Ferro, V.A.; Drummond, R.M. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar] [CrossRef]
- Ruan, J.; Yan, J.; Zheng, D.; Sun, F.; Wang, J.; Han, L.; Zhang, Y.; Wang, T. Comprehensive Chemical Profiling in the Ethanol Extract of Pluchea indica Aerial Parts by Liquid Chromatography/Mass Spectrometry Analysis of Its Silica Gel Column Chromatography Fractions. Molecules 2019, 24, 2784. [Google Scholar] [CrossRef] [Green Version]
- Che, Y.; Wang, Z.; Zhu, Z.; Ma, Y.; Zhang, Y.; Gu, W.; Zhang, J.; Rao, G. Simultaneous Qualitation and Quantitation of Chlorogenic Acids in Kuding Tea Using Ultra-High-Performance Liquid Chromatography-Diode Array Detection Coupled with Linear Ion Trap-Orbitrap Mass Spectrometer. Molecules 2016, 21, 1728. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Delacroix, S.; Chokka, R.; Worthley, S. Hypertension: Pathophysiology and treatment. J. Neurol. Neurophysiol. 2014, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S. Clinical practice guidelines for the management of hypertension in the community: A statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hypertens. 2014, 16, 14–26. [Google Scholar] [CrossRef]
- Sinha, A.D.; Agarwal, R. Clinical Pharmacology of Antihypertensive Therapy for the Treatment of Hypertension in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 757–764. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. Individualization of antihypertensive drug treatment. Diabetes Care 2013, 36, S301–S306. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.E.; Bellamy, M.C. Antihypertensive drugs. Bja Educ. 2015, 15, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Al Disi, S.S.; Anwar, M.A.; Eid, A.H. Anti-hypertensive Herbs and their Mechanisms of Action: Part I. Front Pharm. 2015, 6, 323. [Google Scholar] [CrossRef]
- Inuwa, I.; Ali, B.H.; Al-Lawati, I.; Beegam, S.; Ziada, A.; Blunden, G. Long-term ingestion of Hibiscus sabdariffa calyx extract enhances myocardial capillarization in the spontaneously hypertensive rat. Exp. Biol. Med. 2012, 237, 563–569. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.O.; Saltzman, E.; Blumberg, J.B. Hibiscus Sabdariffa L. Tea (Tisane) Lowers Blood Pressure in Prehypertensive and Mildly Hypertensive Adults–4. J. Nutr. 2009, 140, 298–303. [Google Scholar] [CrossRef]
- Mojiminiyi, F.B.; Audu, Z.; Etuk, E.U.; Ajagbonna, O.P. Attenuation of salt-induced hypertension by aqueous calyx extract of Hibiscus sabdariffa. Niger. J. Physiol. Sci. 2012, 27, 195–200. [Google Scholar]
- Xu, Y.C.; Leung, S.W.S.; Leung, G.P.H.; Man, R.Y.K. Kaempferol enhances endothelium-dependent relaxation in the porcine coronary artery through activation of large-conductance Ca(2+) -activated K(+) channels. Br. J. Pharm. 2015, 172, 3003–3014. [Google Scholar] [CrossRef] [Green Version]
- Błaszczak, W.; Jeż, M.; Szwengiel, A. Polyphenols and inhibitory effects of crude and purified extracts from tomato varieties on the formation of advanced glycation end products and the activity of angiotensin-converting and acetylcholinesterase enzymes. Food Chem. 2020, 314, 126181. [Google Scholar] [CrossRef]
- Tamaroh, S.; Raharjo, S.; Murdiati, A.; Anggrahini, S. Total phenolic content and antioxidant activity of anthocyanin extract from purple yam (Dioscorea alata L.) flour using different solvents. Pak. J. Nutr. 2018, 17, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Shweta, S.; Ritesh, T.; Khadabadi, S.S.; Deokate, U.A. In vitro antioxidant activity and total phenolic, flavonoid contents of the crude extracts of Pterospermum acerifolium Willd leaves (Sterculiaceae). J. Chem. Pharm. Res. 2010, 2, 417–423. [Google Scholar]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Salem, M.; Bernach, M.; Bajdzienko, K.; Giavalisco, P. A Simple Fractionated Extraction Method for the Comprehensive Analysis of Metabolites, Lipids, and Proteins from a Single Sample. J. Vis. Exp. Jove 2017, 124, e55802. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |






| The Tested Sample | IC50 (µg/mL) |
|---|---|
| H1 | 6.293 ± 0.03896 |
| H2 | 0.01255 ± 0.00343 |
| H3 | 0.2058 ± 0.05045 |
| H4 | 9.217 ± 1.0150 |
| H5 | 0.6390 ± 0.032 |
| H6 | >200 |
| H7 | 6.058 ± 0.084 |
| H8 | 8.025 ± 1.501 |
| Captopril standard drug | 0.210 ± 0.005 |
| Peak No. | RT | Formula | [M − H]− | rdb | Error | Mass Fragmentation | The Identified Compounds |
|---|---|---|---|---|---|---|---|
| 1 | 0.74 | C6H7O8 | 207.01407 | 3.5 | 2.543 | 189, 127 | Hydroxy citric acid |
| 2 | 1.48 | C10H16O8 | 263.04074 | 4.5 | 3.73 | 221.03,203.02,189, 185.01,127 | Hibiscus acid hydroxyethyldimethylesther |
| 3 | 2.64 | C8H11O8 | 235.04575 | 3.5 | 3.856 | 189.0, 169.01, 127 | Hibiscus acid hydroxyethylesther |
| 4 | 3.12 | C18H17O14 | 475.07144 | 10.5 | −1.469 | 405.03,363.06,285.02, 235.05,217.03,199.02,189,152.98,111.04 | Kaempferol-3-O-glucuronic acid methyl esther |
| 5 | 3.91 | C9H14O8 | 249.0614 | 6.5 | −3.069 | 307.02, 249.06,206.97, 203.02, 185.01, 127 | Trimethylhydroxycitric acid |
| 6 | 4.23 | C16H17O9 | 353.08823 | 8.5 | 4.309 | 347.06, 305.07, 191.06, 112.98 | Caffeoylquinic acid |
| 7 | 4.46 | C9H14O8 | 249.06 | 6.5 | −3.069 | 307.02, 249.06,206.97, 203.02, 185.01, 127 | Trimethylhydroxycitric acid isomer |
| 8 | 4.60 | C26H27O16 | 595.1319 | 11.5 | −1.1 | 521.11,485.51,419.05,334.04,300.03,249.06,217.03,189, | Delphinidin 3-sambubioside (Hibiscin) |
| 9 | 4.71 | C9H14O8 | 249.0614 | 6.5 | −3.069 | 307.02, 249.06,206.97, 203.02, 185.01, 127 | Trimethylhydroxycitric acid isomer |
| 10 | 5.01 | C16H17O9 | 353.08826 | 8.5 | 4.394 | 263.08, 217.03, 191.06, 145.05 | Caffeoylquinic acid isomer |
| 11 | 5.17 | C16H17O9 | 353.08823 | 8.5 | 4.039 | 191.06, 135.04 | Caffeoylquinic acid isomer |
| 12 | 5.18 | C15H14O11 | 369.0464 | 4.6 | 1.10 | 189, 135,127 | 2-O-trans-Caffeoyl-hydroxycitric acid |
| 13 | 5.42 | C21H19O11 | 447.05923 | 13.5 | 7.667 | 369.08, 299.02, 189, 179.03, 135.04, 112.98 | Kaempferol hexoside |
| 14 | 5.60 | C17H19O9 | 367.10385 | 8.5 | 4.063 | 191.06, 161.02, 133.03 | Methyl chlorogenate |
| 15 | 5.66 | C8H9O7 | 217.03494 | 4.5 | 3.045 | 199.02, 189, 152.98, 111.04 | Hibiscus acid dimethylesther |
| 16 | 5.84 | C16H17O8 | 337.09329 | 8.5 | 4.438 | 304.91, 242.94, 214.93, 193.05, 189, 173.04, 163.04, 112.98 | 3-O-p-Coumaroylquinic acid |
| 17 | 5.94 | C26H27O17 | 611.1259 | 6.5 | −3.069 | 317, 315 | Myricetin 3-arabinogalactoside |
| 18 | 6.28 | C17H19O9 | 367.10382 | 8.5 | 3.981 | 362.88, 174.96, 161.02, 133.03, 112.98 | Methyl chlorogenate (isomer) |
| 19 | 6.36 | C21H18O12 | 461.0750 | 6.6 | 2.19 | 285.1, 180 | Kaempferol 3-O-glucuronide |
| 20 | 6.39 | C16H15O8 | 335.07773 | 9.5 | 4.734 | 248.96, 214.93, 174.96, 161.02, 133.03 | Caffeoylshikimic acid |
| 21 | 6.53 | C16H15O8 | 335.07764 | 9.5 | 4.465 | 304.91, 214.93, 174.96, 161.02, 112.98 | Caffeoylshikimic acid isomer |
| 22 | 6.61 | C17H19O9 | 367.10388 | 8.5 | 4.144 | 135, 179 | Methyl chlorogenate (isomer) |
| 23 | 6.63 | C18H21O9 | 381.11957 | 8.5 | 4.097 | 360.06, 316.95, 206.97, 174.96 | Ethylchlorogenate |
| 24 | 6.85 | C27H30O16 | 609.14734 | 5.6 | 4.144 | 486.14, 367.10,300.03, 248.96, 214.93, 189, 174.96 | Delphinidin 3-neohesperidoside |
| 25 | 7.08 | C21H19O12 | 463.08905 | 12.5 | 4.206 | 304.91, 300.03, 271.03, 207.07, 189.0, 174.96, 129.97 | Delphinidin 3-O-galactoside |
| 26 | 7.24 | C18H21O9 | 381.11957 | 8.5 | 4.097 | 360.06, 316.95, 206.97, 174.96 | Ethylchlorogenate isomer |
| 27 | 7.41 | C27H29O15 | 593.15186 | 13.5 | 2.973 | 486.07, 381.13, 316.95, 285.04, 214.93, 189.0, 74.96, 129.97, 112.98 | Kaempferol 3-alpha-d-glucoside-7-rhamnoside |
| 28 | 7.51 | C18H21O9 | 381.11957 | 8.5 | 4.097 | 360.06, 316.95,206.97, 174.96 | Ethylchlorogenate isomer |
| 29 | 8.17 | C15H10O8 | 317.0302 | 3.6 | −2.90 | 248.9644, 189.0064 | Myricetin |
| 30 | 8.53 | C10H9O4 | 193.05009 | 6.5 | 2.874 | 174.96,133.03 | Ferulic acid |
| 31 | 8.68 | C23H22O12 | 489.1062 | 1.9 | 2.67 | 301.12 | 4′′-O-Acetylquercitrin |
| 32 | 8.78 | C20H18O8 | 385.0931 | 2.9 | −1.1 | Cleomiscosin | |
| 33 | 8.86 | C30H26O14 | 609.1260 | 10.1 | 2.81 | 301.01 | Prodelphinidin B3 |
| 34 | 9.30 | C20H18O8 | 385.0931 | 7.1 | 2.98 | Cleomiscosin isomer | |
| 35 | 9.48 | C15H9O7 | 301.03558 | 11.5 | 4.322 | 260.93, 235.93, 206.93, 174.96, 121.03, 116.93 | Delphinidin |
| 36 | 12.21 | C24H37O13 | 533.22485 | 6.5 | 3.718 | 486.25,379.16,339.20,311.17,183.01 | Dimethyl-delphinidin-glucosyl acetate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.A.; Michel, H.E.; Ezzat, M.I.; Okba, M.M.; EL-Desoky, A.M.; Mohamed, S.O.; Ezzat, S.M. Optimization of an Extraction Solvent for Angiotensin-Converting Enzyme Inhibitors from Hibiscus sabdariffa L. Based on Its UPLC-MS/MS Metabolic Profiling. Molecules 2020, 25, 2307. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102307
Salem MA, Michel HE, Ezzat MI, Okba MM, EL-Desoky AM, Mohamed SO, Ezzat SM. Optimization of an Extraction Solvent for Angiotensin-Converting Enzyme Inhibitors from Hibiscus sabdariffa L. Based on Its UPLC-MS/MS Metabolic Profiling. Molecules. 2020; 25(10):2307. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102307
Chicago/Turabian StyleSalem, Mohamed A., Haidy E. Michel, Marwa I. Ezzat, Mona M. Okba, Ahmed M. EL-Desoky, Shanaz O. Mohamed, and Shahira M. Ezzat. 2020. "Optimization of an Extraction Solvent for Angiotensin-Converting Enzyme Inhibitors from Hibiscus sabdariffa L. Based on Its UPLC-MS/MS Metabolic Profiling" Molecules 25, no. 10: 2307. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102307