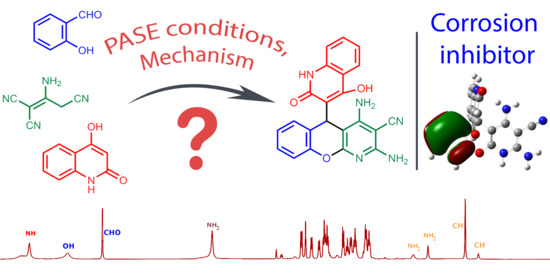
Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism
Abstract
:1. Introduction
2. Results and Discussion
2.1. PASE Approach
2.2. Quantum Chemical Investigation
2.2.1. Quantum Chemistry Approach
2.2.2. Gas Phase Calculations
2.2.3. Solvated Form Calculations
2.2.4. Protonated form Calculations
3. Materials and Methods
3.1. Synthesis
3.1.1. General Information
3.1.2. Synthesis of 4a–i
3.1.3. Isolation of the Intermediate 5
3.1.4. Synthesis of Chromeno[2,3-b]pyridine 4a from 2-(amino-(2-imino-2H-chromen-3-yl)methylene)malononitrile 5 and 4-hydroxyquinolin-2(1H)-one 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chemie Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Nair, V.; Rajesh, C.; Vinod, A.U.; Bindu, S.; Sreekanth, A.R.; Mathen, J.S.; Balagopal, L. Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc. Chem. Res. 2003, 36, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.-P.; Liu, Y. Multicomponent reactions promoted by organosilicon reagents. Curr. Org. Chem. 2011, 15, 2758–2773. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Atom economy—A challenge for organic synthesis: Homogeneous catalysis leads the way. Angew. Chemie Int. Ed. English 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Clarke, P.A.; Santos, S.; Martin, W.H.C. Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem. 2007, 9, 438–440. [Google Scholar] [CrossRef] [Green Version]
- Newhouse, T.; Baran, P.S.; Hoffmann, R.W. The economies of synthesis. Chem. Soc. Rev. 2009, 38, 3010–3021. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Khasanov, A.F.; Kopchuk, D.S.; Kim, G.A.; Slepukhin, P.A.; Kovalev, I.S.; Santra, S.; Zyryanov, G.V.; Majee, A.; Chupakhin, O.N.; Charushin, V.N. Pot, Atom, Step Economic (PASE) approach towards (Aza)-2, 2′-Bipyridines: Synthesis and photophysical studies. ChemistrySelect 2018, 3, 340–347. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, W.B. Pot, Atom, and Step Economy (PASE) Synthesis; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783030225964. [Google Scholar]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef] [PubMed]
- Moseev, T.D.; Varaksin, M.V.; Gorlov, D.A.; Nikiforov, E.A.; Kopchuk, D.S.; Starnovskaya, E.S.; Khasanov, A.F.; Zyryanov, G.V.; Charushin, V.N.; Chupakhin, O.N. Direct CH/CLi coupling of 1,2,4-triazines with C6F5Li followed by aza-Diels-Alder reaction as a pot, atom, and step economy (PASE) approach towards novel fluorinated 2,2′-bipyridine fluorophores. J. Fluor. Chem. 2019, 224, 89–99. [Google Scholar] [CrossRef]
- Chen, X.-B.; Xiong, S.-L.; Xie, Z.-X.; Wang, Y.-C.; Liu, W. Three-Component One-Pot Synthesis of Highly Functionalized Bis-Indole Derivatives. ACS omega 2019, 4, 11832–11837. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Nasybullin, R.F.; Vereshchagin, A.N.; Egorov, M.P. Fast Efficient and General PASE Approach to Medicinally Relevant 4H,5H-Pyrano-[4,3-b]pyran-5-one and 4,6-Dihydro-5H-pyrano-[3,2-c]pyridine-5-one Scaffolds. Helv. Chim. Acta 2016, 99, 724–731. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Illa, J.; Roca, J.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 32 to 36 °C temperature range. Mater. Corros. 2001, 52, 140–146. [Google Scholar] [CrossRef]
- NACE—Economic Impact, Assessment of the Global Cost of Corrosion. Available online: http://impact.nace.org/economic-impact.aspx (accessed on 18 January 2020).
- U.S. Report on Corrosion Costs and Preventive Strategies in the United States. Available online: http://www.corrosioncost.com (accessed on 18 January 2020).
- Bristol, J.A.; Gold, E.H.; Gross, I.; Lovey, R.G.; Long, J.F. Gastric antisecretory agents. 2. Antisecretory activity of 9-[(aminoalkyl)thio]-9H-xanthenes and 5-[(aminoalkyl)thio]-5H-[1]benzopyrano-[2,3-b]pyridines. J. Med. Chem. 1981, 24, 1010–1013. [Google Scholar] [CrossRef]
- Anderson, D.R.; Hegde, S.; Reinhard, E.; Gomez, L.; Vernier, W.F.; Lee, L.; Liu, S.; Sambandam, A.; Snider, P.A.; Masih, L. Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2005, 15, 1587–1590. [Google Scholar] [CrossRef]
- Tu, X.-J.; Fan, W.; Hao, W.-J.; Jiang, B.; Tu, S.-J. Three-component bicyclization providing an expedient access to Pyrano[2′,3′:5,6]pyrano[2,3-b]Pyridines and its derivatives. ACS Comb. Sci. 2014, 16, 647–651. [Google Scholar] [CrossRef]
- Abdelmoniem, A.M.; Ghozlan, S.A.S.; Abdelmoniem, D.M.; Elwahy, A.H.M.; Abdelhamid, I.A. Facile one-pot, three-component synthesis of novel Bis-Heterocycles incorporating 5H-chromeno[2,3-b]pyridine-3-carbonitrile Derivatives. J. Heterocycl. Chem. 2017, 54, 2844–2849. [Google Scholar] [CrossRef]
- Bardasov, I.N.; Alekseeva, A.U.; Mihailov, D.L.; Ershov, O.V.; Grishanov, D.A. Double heteroannulation reactions of 1-naphthol with alkyl-and arylmethylidene derivatives of malononitrile dimer. Tetrahedron Lett. 2015, 56, 1830–1832. [Google Scholar] [CrossRef]
- Olyaei, A.; Shahsavari, M.S.; Sadeghpour, M. Organocatalytic approach toward the green one-pot synthesis of novel benzo[f]chromenes and 12H-benzo[5,6]chromeno[2,3-b]pyridines. Res. Chem. Intermed. 2018, 44, 943–956. [Google Scholar] [CrossRef]
- Olyaei, A.; Shafie, Z.; Sadeghpour, M. An efficient and one-pot green synthesis of novel 6-oxo-7-aryl-6,7-dihydrochromeno pyrano[2,3-b]pyridine derivatives. Tetrahedron Lett. 2018, 59, 3567–3570. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Mao, J.; Hu, L.; Wu, X.; Guo, C. Three-component domino cyclization of novel carbazole and indole fused pyrano[2,3-c]pyridine derivatives. Tetrahedron Lett. 2016, 57, 1985–1989. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Egorov, M.P. Efficient Multicomponent Approach to the Medicinally Relevant 5-aryl-chromeno[2,3-b]pyridine Scaffold. Polycycl. Aromat. Compd. 2020, 40, 108–115. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Selective multicomponent ‘one-pot’ approach to the new 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)chromeno[2,3-b]pyridine scaffold in pyridine-ethanol catalyst/solvent system. Monatshefte für Chemie Chem. Mon. 2019, 150, 1073–1078. [Google Scholar] [CrossRef]
- Elinson, M.; Vereshchagin, A.; Anisina, Y.; Krymov, S.; Fakhrutdinov, A.; Egorov, M. Potassium fluoride catalysed multicomponent approach to medicinally privileged 5-[3-hydroxy-6-(hydroxymethyl)-4H-pyran-2-yl] substituted chromeno[2,3-b]pyridine scaffold. Arkivoc 2019, part ii, 38–49. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Korolev, V.A.; Egorov, M.P. Pot, atom and step-economic (PASE) synthesis of medicinally relevant spiro [oxindole-3, 4′-pyrano [4, 3-b] pyran] scaffold. Heterocycl. Commun. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Elinson, M.N.; Nasybullin, R.F.; Ryzhkov, F.V.; Egorov, M.P. Solvent-free and ‘on-water’ multicomponent assembling of salicylaldehydes, malononitrile and 3-methyl-2-pyrazolin-5-one: A fast and efficient route to the 2-amino-4-(1H-pyrazol-4-yl)-4H-chromene scaffold. Comptes Rendus Chim. 2014, 17, 437–442. [Google Scholar] [CrossRef]
- Elinson, M.N.; Nasybullin, R.F.; Ryzhkov, F.V.; Zaimovskaya, T.A.; Nikishin, G.I. Solvent-free and ‘on-water’ multicomponent assembling of aldehydes, 3-methyl-2-pyrazoline-5-one, and malononitrile: Fast and efficient approach to medicinally relevant pyrano[2,3-c]pyrazole scaffold. Monatshefte für Chemie Chem. Mon. 2015, 146, 631–635. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V. V Organic synthesis “On Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. PASE facile and efficient multicomponent approach to the new type of 5-C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2018, 28, 372–374. [Google Scholar] [CrossRef]
- Elinson, M.N.; Gorbunov, S.V.; Vereshchagin, A.N.; Nasybullin, R.F.; Goloveshkin, A.S.; Bushmarinov, I.S.; Egorov, M.P. Chemical and electrocatalytic cascade cyclization of salicylaldehyde with three molecules of malononitrile: ‘one-pot’ simple and efficient way to the chromeno[2,3-b]pyridine scaffold. Tetrahedron 2014, 70, 8559–8563. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Amer, A.A. Synthesis of Some New Polyfunctionalized Pyridines. J. Heterocycl. Chem. 2018, 55, 297–301. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; el taweel, M.; Elmougi, S.; Elagamey, A. The condensation of active methylene reagents with salicylaldehyde: Novel synthesis of chromene, azaanthracene, pyrano[3,4-c]chromene and chromeno[3,4-c]pyridine derivatives. J. Heterocycl. Chem. 2004, 41, 655–658. [Google Scholar] [CrossRef]
- Sakurai, A.; Midorikawa, H.; Hashimoto, Y. The cyclization of ethyl cyanoacetate and salicylaldehyde or 3-methoxysalicylaldehyde with ketones by means of ammonium acetate. Bull. Chem. Soc. Jpn. 1970, 43, 2925–2933. [Google Scholar] [CrossRef] [Green Version]
- Soleiman, H.A.; Koraiem, A.I.M.; Mahmoud, N.Y. Synthesis of new fused hetecrocyclic compounds of benzpyrid-4-one derivatives and their some biological activity. J. Chin. Chem. Soc. 2004, 51, 553–560. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Al-Mayouf, A.M.; Al-Ameery, A.K.; Al-Suhybani, A.A. Inhibition of type 304 stainless steel corrosion in 2 M sulfuric acid by some benzoazoles—Time and temperature effects. Corrosion 2001, 57, 614–620. [Google Scholar] [CrossRef]
- Lashgari, M.; Arshadi, M. DFT studies of pyridine corrosion inhibitors in electrical double layer: Solvent, substrate, and electric field effects. Chem. Phys. 2004, 299, 131–137. [Google Scholar] [CrossRef]
- Levin, M.; Wiklund, P.; Leygraf, C. Bioorganic compounds as copper corrosion inhibitors in hydrocarbon media. Corros. Sci. 2012, 58, 104–114. [Google Scholar] [CrossRef]
- Paul, P.K.; Saraswat, V.; Yadav, M. Chromenes as efficient corrosion inhibitor for mild steel in HCl solution. J. Adhes. Sci. Technol. 2019, 33, 1275–1293. [Google Scholar] [CrossRef]
- Khaled, K.; Al-Mobarak, N.A. A predictive model for corrosion inhibition of mild steel by thiophene and its derivatives using artificial neural network. Int. J. Electrochem. Sci. 2012, 7, 1045–1059. [Google Scholar]
- Taylor, C.D. Design and prediction of corrosion inhibitors from quantum chemistry. J. Electrochem. Soc. 2015, 162, 340–346. [Google Scholar] [CrossRef]
- Taylor, C.D.; Chandra, A.; Vera, J.; Sridhar, N. Design and prediction of corrosion inhibitors from quantum chemistry II. A general framework for prediction of effective oil/water partition coefficients and speciation from quantum chemistry. J. Electrochem. Soc. 2015, 162, 347–353. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Kabanda, M.M.; Murulana, L.C.; Singh, A.K.; Shukla, S.K. Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 12940–12958. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Khaled, K.; Fadl-allah, S.; Hammouti, B. Some benzotriazole derivatives as corrosion inhibitors for copper in acidic medium: Experimental and quantum chemical molecular dynamics approach. Mater. Chem. Phys. 2009, 117, 148–155. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Yu, W.; Yan, Y.; You, L.; Liu, L. Molecular modeling of the inhibition mechanism of 1-(2-aminoethyl)-2-alkyl-imidazoline. Corros. Sci. 2010, 52, 2059–2065. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T. Quantum chemical evaluation of the corrosion inhibition of novel aromatic hydrazide derivatives on mild steel in hydrochloric acid. Comput. Theor. Chem. 2016, 1093, 73–80. [Google Scholar] [CrossRef]
- Dega-Szafran, Z.; Kania, A.; Nowak-Wydra, B.; Szafran, M. UV, 1H and 13C NMR spectra, and AM1 studies of protonation of aminopyridines. J. Mol. Struct. 1994, 322, 223–232. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria., G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Becke’s three parameter hybrid method using the LYP correlation functional. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Metri, N.; Sallenave, X.; Plesse, C.; Beouch, L.; Aubert, P.-H.; Goubard, F.; Chevrot, C.; Sini, G. Processable star-shaped molecules with triphenylamine core as hole-transporting materials: Experimental and theoretical approach. J. Phys. Chem. C 2012, 116, 3765–3772. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–i are available from the authors. |








| Entry | Solvent | Catalyst | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Solvent-free | - | 2 | 80 | 17 |
| 2 | Solvent-free | KF | 2 | 80 | 25 |
| 3 | Solvent-free | AcONa | 2 | 80 | 37 |
| 4 | H2O | - | 2 | 80 | 16 |
| 5 | H2O | AcONa | 2 | 80 | 31 |
| 6 | EtOH | - | 2 | 78 | 19 2 |
| 7 | EtOH | AcONa | 2 | 78 | 35 2 |
| 8 | EtOH | Et3N | 2 | 78 | 63 2 |
| 9 | EtOH | Morph | 2 | 78 | 57 2 |
| 10 | Py | - | 2 | 116 | 61 2 |
| 11 | EtOH-Py (4:1) | - | 2 | 78 | 87 2 |
| 12 | EtOH-Py (3:1) | - | 2 | 78 | 95 2 |
| 13 | EtOH-Py (2:1) | - | 2 | 78 | 92 2 |
| 14 | EtOH-Py (3:1) | - | 1 | 78 | 89 2 |
 |
 |
| Compound | 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i |
|---|---|---|---|---|---|---|---|---|---|
| Gas Phase Calculations | |||||||||
| Total energy, a.u. | −1347.19 | −1386.52 | −1461.75 | −1501.07 | −4035.28 | −1806.81 | −3920.74 | −1551.75 | −1500.87 |
| E(HOMO), eV | −5.707 | −5.649 | −5.644 | −5.558 | −5.695 | −5.835 | −5.830 | −6.065 | −5.669 |
| E(LUMO), eV | −2.040 | −2.023 | −1.997 | −1.982 | −2.072 | −2.135 | −2.131 | −2.416 | −2.047 |
| ΔE(L-H), eV | 3.667 | 3.626 | 3.647 | 3.576 | 3.623 | 3.700 | 3.699 | 3.649 | 3.622 |
| μ, D | 7.896 | 8.071 | 7.645 | 8.119 | 7.487 | 7.325 | 7.351 | 7.619 | 7.670 |
| χ | 3.874 | 3.836 | 3.821 | 3.770 | 3.884 | 3.986 | 3.981 | 4.241 | 3.858 |
| η | 1.834 | 1.813 | 1.824 | 1.788 | 1.812 | 1.85 | 1.849 | 1.825 | 1.811 |
| ω | 4.092 | 4.058 | 4.003 | 3.975 | 4.164 | 4.301 | 4.286 | 4.929 | 4.109 |
| σ | 0.545 | 0.552 | 0.548 | 0.559 | 0.552 | 0.541 | 0.541 | 0.548 | 0.552 |
| Calculations for Solvated Compounds | |||||||||
| Total energy, a.u. | −1347.22 | −1386.55 | −1461.77 | −1501.10 | −4035.31 | −1806.84 | −3920.76 | −1551.78 | −1500.90 |
| E(HOMO), eV | −5.993 | −5.947 | −5.940 | −5.900 | −5.968 | −6.037 | −6.035 | −6.139 | −5.934 |
| E(LUMO), eV | −1.795 | −1.791 | −1.783 | −1.791 | −1.822 | −1.826 | −1.825 | −2.726 | −1.810 |
| ΔE(L-H), eV | 4.198 | 4.156 | 4.157 | 4.109 | 4.146 | 4.211 | 4.210 | 3.413 | 4.124 |
| μ, D | 10.263 | 11.789 | 9.883 | 10.873 | 11.137 | 9.490 | 9.486 | 9.609 | 10.135 |
| χ | 3.894 | 3.869 | 3.867 | 3.846 | 3.895 | 3.932 | 3.930 | 4.433 | 3.872 |
| η | 2.099 | 2.078 | 2.079 | 2.055 | 2.073 | 2.106 | 2.105 | 1.707 | 2.062 |
| ω | 3.612 | 3.602 | 3.596 | 3.599 | 3.659 | 3.671 | 3.669 | 5.756 | 3.635 |
| σ | 0.476 | 0.481 | 0.481 | 0.487 | 0.482 | 0.475 | 0.475 | 0.586 | 0.485 |
| Calculations for protonated forms of studied compounds | |||||||||
| Total energy, a.u. | −1347.67 | −1386.99 | −1462.22 | −1501.55 | −4035.76 | −1807.29 | −3921.21 | −1552.22 | −1501.34 |
| E(HOMO), eV | −6.592 | −6.541 | −6.374 | −6.350 | −6.449 | −6.623 | −6.617 | −6.670 | −6.321 |
| E(LUMO), eV | −1.983 | −1.971 | −1.967 | −1.977 | −2.026 | −2.035 | −2.033 | −2.927 | −2.044 |
| ΔE(L-H), eV | 4.609 | 4.57 | 4.407 | 4.373 | 4.423 | 4.588 | 4.584 | 3.743 | 4.277 |
| μ, D | 9.704 | 9.785 | 11.099 | 8.722 | 13.356 | 12.543 | 13.784 | 16.319 | 11.489 |
| χ | 4.288 | 4.256 | 4.171 | 4.164 | 4.263 | 4.329 | 4.325 | 4.799 | 4.183 |
| η | 2.305 | 2.285 | 2.204 | 2.187 | 2.212 | 2.294 | 2.292 | 1.872 | 2.139 |
| ω | 3.988 | 3.964 | 3.947 | 3.964 | 4.108 | 4.085 | 4.081 | 6.151 | 4.090 |
| σ | 0.434 | 0.438 | 0.454 | 0.457 | 0.452 | 0.436 | 0.436 | 0.534 | 0.468 |
| Compound | HOMO | LUMO |
|---|---|---|
| In Gas Phase | ||
| 4a |  |  |
| 4e |  |  |
| 4h |  |  |
| Solvated Molecules | ||
| 4c |  |  |
| Protonated Molecules | ||
| 4a |  |  |
| 4h |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112573
Ryzhkov FV, Ryzhkova YE, Elinson MN, Vorobyev SV, Fakhrutdinov AN, Vereshchagin AN, Egorov MP. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules. 2020; 25(11):2573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112573
Chicago/Turabian StyleRyzhkov, Fedor V., Yuliya E. Ryzhkova, Michail N. Elinson, Stepan V. Vorobyev, Artem N. Fakhrutdinov, Anatoly N. Vereshchagin, and Mikhail P. Egorov. 2020. "Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism" Molecules 25, no. 11: 2573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25112573