Stabilization of the Highly Hydrophobic Membrane Protein, Cytochrome bd Oxidase, on Metallic Surfaces for Direct Electrochemical Studies
Abstract
:1. Introduction
2. Results and Discussion
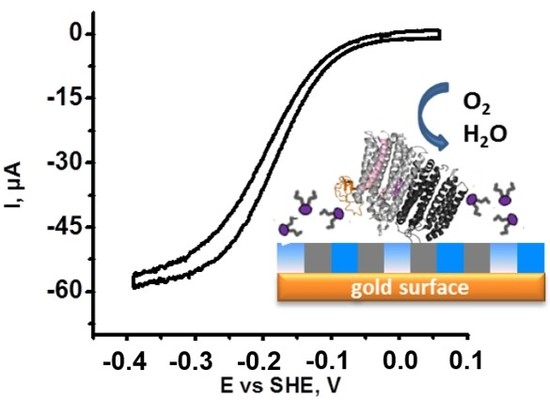
2.1. Identification of Protein Stability and Analysis of the Catalytic Response
- (i)
- The half wave potential Ecat of the sigmoidal curve, which is related to the kinetic efficiency of the oxygen reaction. The higher Ecat, the lower the overpotential for O2 reduction.
- (ii)
- The slope Δi/ΔE of the catalytic curve in the region of limiting current, which provides information on the distribution of the orientations of the proteins on the electrode surface [23].
- (iii)
- The variability Δi/i of the limiting current value between two consecutive scans separated by 10 min that allows the evaluation of the stability of the protein films.
2.2. Requirement of Lipids for Stabilization of the Protein Film
2.3. Mutual Influence of Lipid Type and Electrode Surface Charge
2.4. Influence of the Length of the Thiol
2.5. Covalent vs. Non-Covalent Attachment of the Protein
3. Materials and Methods
3.1. Chemicals
3.2. Protein Preparation
3.3. Electrode Modification for Non-Covalent Attachment of the Protein
3.4. Electrode Modification for Covalent Attachment of the Protein
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, H.; Flynn, A.D. Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng. 2016, 18, 51–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arinaminpathy, Y.; Khurana, E.; Engelman, D.M.; Gerstein, M.B. Computational analysis of membrane proteins: The largest class of drug targets. Drug Discov. Today 2009, 14, 1130–1135. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 62–87. [Google Scholar] [CrossRef] [Green Version]
- Robinson, N.C.; Zborowski, J.; Talbert, L.H. Cardiolipin-depleted bovine heart cytochrome c oxidase: Binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry 1990, 29, 8962–8969. [Google Scholar] [CrossRef]
- Wenz, T.; Hielscher, R.; Hellwig, P.; Schägger, H.; Richers, S.; Hunte, C. Role of phospholipids in respiratory cytochrome bc1 complex catalysis and supercomplex formation. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1787, 609–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jünemann, S. Cytochrome bd terminal oxidase1. Biochim. Biophys. Acta 1997, 1321, 107–127. [Google Scholar] [CrossRef] [Green Version]
- Borisov, V.B.; Gennis, R.B.; Hemp, J.; Verkhovsky, M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta 2011, 1807, 1398–1413. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Silaghi-Dumitrescu, R.; Ljungdahl, L.G.; Kurtz, D.M. Cytochrome bd Oxidase, Oxidative Stress, and Dioxygen Tolerance of the Strictly Anaerobic Bacterium Moorella thermoacetica. J. Bacteriol. 2005, 187, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Borisov, V.B.; Forte, E.; Davletshin, A.; Mastronicola, D.; Sarti, P.; Giuffrè, A. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 2013, 587, 2214–2218. [Google Scholar] [CrossRef]
- Giuffrè, A.; Borisov, V.B.; Arese, M.; Sarti, P.; Forte, E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta 2014, 1837, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meunier, B.; Madgwick, S.A.; Reil, E.; Oettmeier, W.; Rich, P.R. New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry 1995, 34, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Hartman, T.E.; Jacobs, W.R. A Mycobacterium tuberculosis Cytochrome bd Oxidase Mutant Is Hypersensitive to Bedaquiline. mBio 2014, 5, e01275-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogi, T.; Ui, H.; Shiomi, K.; Ōmura, S.; Miyoshi, H.; Kita, K. Antibiotics LL-Z1272 identified as novel inhibitors discriminating bacterial and mitochondrial quinol oxidases. Biochim. Biophys. Acta 2009, 1787, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Dueweke, T.J.; Gennis, R.B. Epitopes of monoclonal antibodies which inhibit ubiquinol oxidase activity of Escherichia coli cytochrome d complex localize functional domain. J. Biol. Chem. 1990, 265, 4273–4277. [Google Scholar]
- Dueweke, T.J.; Gennis, R.B. Proteolysis of the cytochrome d complex with trypsin and chymotrypsin localizes a quinol oxidase domain. Biochemistry 1991, 30, 3401–3406. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murai, M.; Fujita, D.; Sakamoto, K.; Miyoshi, H.; Yoshida, M.; Mogi, T. Mass Spectrometric Analysis of the Ubiquinol-binding Site in Cytochrome bd from Escherichia coli. J. Biol. Chem. 2006, 281, 1905–1912. [Google Scholar] [CrossRef] [Green Version]
- Mogi, T.; Akimoto, S.; Endou, S.; Watanabe-Nakayama, T.; Mizuochi-Asai, E.; Miyoshi, H. Probing the Ubiquinol-Binding Site in Cytochrome bd by Site-Directed Mutagenesis. Biochemistry 2006, 45, 7924–7930. [Google Scholar] [CrossRef]
- Safarian, S.; Rajendran, C.; Müller, H.; Preu, J.; Langer, J.D.; Ovchinnikov, S.; Hirose, T.; Kusumoto, T.; Sakamoto, J.; Michel, H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science 2016, 352, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Safarian, S.; Hahn, A.; Mills, D.J.; Radloff, M.; Eisinger, M.L.; Nikolaev, A.; Meier-Credo, J.; Melin, F.; Miyoshi, H.; Gennis, R.B.; et al. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science 2019, 366, 100–104. [Google Scholar] [CrossRef]
- Theßeling, A.; Rasmussen, T.; Burschel, S.; Wohlwend, D.; Kägi, J.; Müller, R.; Böttcher, B.; Friedrich, T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 2019, 10, 5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, E.; Nikolaev, A.; Nasiri, H.R.; Hoeser, J.; Friedrich, T.; Hellwig, P.; Melin, F. Creation of a gold nanoparticle based electrochemical assay for the detection of inhibitors of bacterial cytochrome bd oxidases. Bioelectrochemistry 2016, 111, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Léger, C.; Jones, A.K.; Albracht, S.P.J.; Armstrong, F.A. Effect of a Dispersion of Interfacial Electron Transfer Rates on Steady State Catalytic Electron Transport in [NiFe]-hydrogenase and Other Enzymes. J. Phys. Chem. B 2002, 106, 13058–13063. [Google Scholar] [CrossRef]
- Shokri, A.; Larsson, G. Characterisation of the Escherichia coli membrane structure and function during fedbatch cultivation. Microb. Cell Fact. 2004, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorence, R.M.; Miller, M.J.; Borochov, A.; Faiman-Weinberg, R.; Gennis, R.B. Effects of pH and detergent on the kinetic and electrochemical properties of the purified cytochrome d terminal oxidase complex of Escherichia coli. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1984, 790, 148–153. [Google Scholar] [CrossRef]
- Kolonay, J.F.; Moshiri, F.; Gennis, R.B.; Kaysser, T.M.; Maier, R.J. Purification and characterization of the cytochrome bd complex from Azotobacter vinelandii: Comparison to the complex from Escherichia coli. J. Bacteriol. 1994, 176, 4177–4181. [Google Scholar] [CrossRef] [Green Version]
- Chi, Q.; Zhang, J.; Andersen, J.E.T.; Ulstrup, J. Ordered Assembly and Controlled Electron Transfer of the Blue Copper Protein Azurin at Gold (111) Single-Crystal Substrates. J. Phys. Chem. B 2001, 105, 4669–4679. [Google Scholar] [CrossRef]
- Murgida, D.H.; Hildebrandt, P. Proton-Coupled Electron Transfer of Cytochrome c. J. Am. Chem. Soc. 2001, 123, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, H.; Khoshtariya, D.E.; Yamamoto, H.; Dick, A.; Waldeck, D.H. Electron-Transfer Dynamics of Cytochrome C: A Change in the Reaction Mechanism with Distance. Angew. Chem. Int. Ed. 2002, 41, 4700–4703. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Nakamura, N.; Ohno, H.; Leigh, B.S.; Niki, K.; Gray, H.B.; Richards, J.H. Mimicking Protein−Protein Electron Transfer: Voltammetry of Pseudomonas aeruginosa Azurin and the Thermus thermophilus CuA Domain at ω-Derivatized Self-Assembled-Monolayer Gold Electrodes. J. Am. Chem. Soc. 2004, 126, 13954–13961. [Google Scholar] [CrossRef]
- Schartner, J.; Güldenhaupt, J.; Mei, B.; Rögner, M.; Muhler, M.; Gerwert, K.; Kötting, C. Universal Method for Protein Immobilization on Chemically Functionalized Germanium Investigated by ATR-FTIR Difference Spectroscopy. J. Am. Chem. Soc. 2013, 135, 4079–4087. [Google Scholar] [CrossRef]
- Ataka, K.; Giess, F.; Knoll, W.; Naumann, R.; Haber-Pohlmeier, S.; Richter, B.; Heberle, J. Oriented Attachment and Membrane Reconstitution of His-Tagged Cytochrome c Oxidase to a Gold Electrode: In Situ Monitoring by Surface-Enhanced Infrared Absorption Spectroscopy. J. Am. Chem. Soc. 2004, 126, 16199–16206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriegel, S.; Uchida, T.; Osawa, M.; Friedrich, T.; Hellwig, P. Biomimetic Environment to Study, E. coli Complex I through Surface-Enhanced IR Absorption Spectroscopy. Biochemistry 2014, 53, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- Grytsyk, N.; Sugihara, J.; Kaback, H.R.; Hellwig, P. pKa of Glu325 in LacY. Proc. Natl. Acad. Sci. USA 2017, 114, 1530–1535. [Google Scholar] [CrossRef] [Green Version]
- Hoeser, J.; Hong, S.; Gehmann, G.; Gennis, R.B.; Friedrich, T. Subunit CydX of Escherichia coli cytochrome bd ubiquinol oxidase is essential for assembly and stability of the di-heme active site. FEBS Lett. 2014, 588, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, C.; Nett, J.H.; Trumpower, B.L.; Hunte, C. Specific roles of protein–phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001, 20, 6591–6600. [Google Scholar] [CrossRef] [Green Version]
- Palsdottir, H.; Hunte, C. Lipids in membrane protein structures. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Al-Attar, S.; Yu, Y.; Pinkse, M.; Hoeser, J.; Friedrich, T.; Bald, D.; de Vries, S. Cytochrome bd Displays Significant Quinol Peroxidase Activity. Sci. Rep. 2016, 6, 27631. [Google Scholar] [CrossRef] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20. [Google Scholar] [CrossRef]




| Immobilization Conditions | Ecat | Δi/ΔE | Δi/i | |||
|---|---|---|---|---|---|---|
| Sample | % Lipid | Lipid Type | Thiol Type | (V) | (μA·mV−1) | |
| 1 | 0 | - | HT/MCH (1/1) | 0.06 | 0.007 | 0.39 |
| 1 | 5 | PE/PG (1/1) | HT/MCH (1/1) | 0.16 | 0.017 | 0.26 |
| 1 | 15 | PE/PG (1/1) | HT/MCH (1/1) | 0.16 | 0.020 | 0.13 |
| 1 | 22 | PE/PG (1/1) | HT/MCH (1/1) | 0.16 | 0.008 | 0.07 |
| 1 | 30 | PE/PG (1/1) | HT/MCH (1/1) | 0.16 | 0.020 | 0.08 |
| 1 | 44 | PE/PG (1/1) | HT/MCH (1/1) | 0.11 | 0.009 | 0.40 |
| 2 | 0 | - | HT/MCH (1/1) | 0.11 | 0.013 | 0.09 |
| 2 | 2.5 | PG | HT/MCH (1/1) | 0.13 | 0.012 | 0.06 |
| 2 | 2.5 | PE | HT/MCH (1/1) | 0.08 | 0.052 | 0.15 |
| 2 | 2.5 | PE | HT/MCH/MPA (1/1/1) | 0.16 | 0.028 | 0.04 |
| 2 | 2.5 | PE | HT/MCH/MHA (1/1/1) | 0.11 | 0.010 | 0.07 |
| 2 | 2.5 | PE | HT/MCH/MUA (1/1/1) | 0.01 | 0.023 | 0.10 |
| 2 | 2.5 | PE | HT/MCH/cyst (1/1/1) | 0.20 | 0.019 | 0.08 |
| 2 | 2.5 | PE | Ni-NTA | 0.12 | 0.052 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, A.; Makarchuk, I.; Thesseling, A.; Hoeser, J.; Friedrich, T.; Melin, F.; Hellwig, P. Stabilization of the Highly Hydrophobic Membrane Protein, Cytochrome bd Oxidase, on Metallic Surfaces for Direct Electrochemical Studies. Molecules 2020, 25, 3240. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25143240
Nikolaev A, Makarchuk I, Thesseling A, Hoeser J, Friedrich T, Melin F, Hellwig P. Stabilization of the Highly Hydrophobic Membrane Protein, Cytochrome bd Oxidase, on Metallic Surfaces for Direct Electrochemical Studies. Molecules. 2020; 25(14):3240. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25143240
Chicago/Turabian StyleNikolaev, Anton, Iryna Makarchuk, Alexander Thesseling, Jo Hoeser, Thorsten Friedrich, Frédéric Melin, and Petra Hellwig. 2020. "Stabilization of the Highly Hydrophobic Membrane Protein, Cytochrome bd Oxidase, on Metallic Surfaces for Direct Electrochemical Studies" Molecules 25, no. 14: 3240. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25143240