Porous V2O5/TiO2 Nanoheterostructure Films with Enhanced Visible-Light Photocatalytic Performance Prepared by the Sparking Method
Abstract
:1. Introduction
2. Results and Discussion
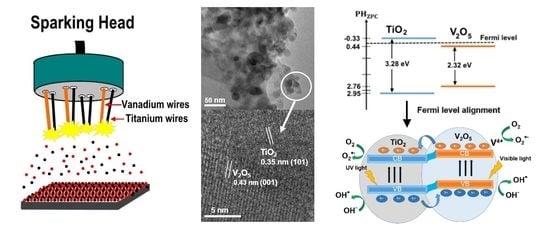
2.1. Morphology, Crystal Structure, and Property Investigations
2.2. UV–Vis Results, Band Gap Investigation, and Photocatalytic Performance
2.3. Possible Photocatalysis Process in the Visible-Light-Irradiated V2O5/TiO2 System
3. Experimental Setup
3.1. Preparation of Samples
3.2. Characterization Methods
3.3. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, R.; Singh, S.; Dalai, M.; Saravanan, M.; Agrawal, V.V.; Srivastava, A.K. Photocatalytic degradation of polypropylene film using TiO2-based nanomaterials under solar irradiation. Mater. Des. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Divya, P.; Arulkumar, S.; Parthiban, S.; Goswami, A.; Ahamade, T.; Gawande, M.B. Rapid and Scalable Wire-bar Strategy for Coating of TiO2 Thin-films: Effect of Post-Annealing Temperatures on Structures and Catalytic Dye-Degradation. Molecules 2020, 25, 1683. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Hu, C.; Qi, W.; An, X.; Liu, H.; Qu, J. Defect-enhanced photocatalytic removal of dimethylarsinic acid over mixed-phase mesoporous TiO2. J. Environ. Sci. 2020, 91, 35–42. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.-E.; Wen, W.; Xia, Y.; Wu, J.-M. Photocatalytic activity of TiO2 nanorods, nanowires and nanoflowers filled with TiO2 nanoparticles. Thin Solid Film. 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Birnal, P.; Marco de Lucas, M.C.; Pochard, I.; Domenichini, B.; Imhoff, L. Photocatalytic properties of atomic layer deposited TiO2 inverse opals and planar films for the degradation of dyes. Appl. Surf. Sci. 2020, 512, 145693. [Google Scholar] [CrossRef]
- Wei, T.; Lau, W.M.; An, X.; Yu, X. Interfacial Charge Transfer in MoS2/TiO2 Heterostructured Photocatalysts: The Impact of Crystal Facets and Defects. Molecules 2019, 24, 1769. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, W.; Jinab, C.; Heab, Q.; Wangab, Y.; Liab, R.; Liab, W. Protected, Email Fabrication of ZIF-8@TiO2 micron composite via hydrothermal method with enhanced absorption and photocatalytic activities in tetracycline degradation. J. Alloys Compd. 2020, 825, 154008. [Google Scholar] [CrossRef]
- Martínez, M.C.N.; Mazierski, P.; Kobylański, M.; Szczepanska, G.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Growth, Structure, and Photocatalytic Properties of Hierarchical V2O5-TiO2 Nanotube Arrays Obtained from the One-step Anodic Oxidation of Ti-V Alloys. Molecules 2017, 22, 580. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Chu, M.; Kim, S.W.; Ryu, J.-W. Optical and electrical properties of V2O5 nanorod films grown using an electron beam. Thin Solid Film. 2013, 547, 198–201. [Google Scholar] [CrossRef]
- Lamoureux, B.; Singh, V.; Jovic, V.; Kuyyalil, J.; Su, T.-Y.; Smith, K. Structural and electronic properties of thermally evaporated V2O5 epitaxial thin films. Thin Solid Film. 2016, 615, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, X.; Zhao, Q.; Ke, J.; Zhang, D. Novel V2O5/BiVO4/TiO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity for the Degradation of Toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.R.; Qiao, L.; Liu, L.X.; Su, Q.; Zhu, C.Q.; Liu, X. Synthesis of one-dimensional TiO2/V2O5 branched heterostructures and their visible light photocatalytic activity towards Rhodamine B. Nanotechnology 2011, 22, 225702. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.; Li, X.; Wang, C. Plasmonic Ag-TiO2−x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B Environ. 2017, 204, 67–77. [Google Scholar] [CrossRef]
- Van Dao, D.; Nguyen, T.T.; Song, H.-Y.; Yang, J.-K.; Kim, T.-W.; Yu, Y.-T.; Lee, I.-H. Ionic liquid-assisted preparation of Ag-CeO2 nanocomposites and their improved photocatalytic activity. Mater. Des. 2018, 159, 186–194. [Google Scholar] [CrossRef]
- Mondal, H.M.; Dutta, S.K. Enhanced photocatalysis performance of mechano-synthesized V2O5–TiO2 nanocomposite for wastewater treatment: Correlation of structure with photocatalytic performance. Mater. Chem. Phys. 2020, 248, 122947. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, Z.; Liu, Y.; Peng, Z.; Zhou, M.; Zhang, C.; Jin, W. Synthesis and photocatalytic property of V2O5@TiO2 core-shell microspheres towards gaseous benzene. Catal. Today 2019, 321, 164–171. [Google Scholar] [CrossRef]
- Choi, S.; Lee, M.-S.; Park, D.-W. Photocatalytic performance of TiO2/V2O5 nanocomposite powder prepared by DC arc plasma. Curr. Appl. Phys. 2014, 14, 433–438. [Google Scholar] [CrossRef]
- Bayati, M.R.; Molaei, R.; Moshfegh, A.Z.; Golestanifard, F. A strategy for single-step elaboration of V2O5-grafted TiO2 nanostructured photocatalysts with evenly distributed pores. J. Alloy. Compd. 2011, 509, 6236–6241. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Ko, W.C.; Kang, S.; Kim, K.M.; Jeong, Y.K. Surface passivation of highly stable TiO2/V2O5 photocatalyst by atomic layer deposited-Al2O3. Appl. Surf. Sci. 2020, 507, 145128. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, S.; Gu, P.; Zhang, T.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020, 578, 402–411. [Google Scholar] [CrossRef]
- Kumpika, T.; Thongsuwan, W.; Singjai, P. Atomic force microscopy imaging of ZnO nanodots deposited on quartz by sparking off different tip shapes. Surf. Interface Anal. 2006, 39, 58–63. [Google Scholar] [CrossRef]
- Thongsuwan, W.; Kumpika, T.; Singjai, P. Photocatalytic property of colloidal TiO2 nanoparticles prepared by sparking process. Curr. Appl. Phys. 2008, 8, 563–568. [Google Scholar] [CrossRef]
- Thongsuwan, W.; Kumpika, T.; Singjai, P. Effect of high roughness on a long aging time of superhydrophilic TiO2 nanoparticle thin films. Curr. Appl. Phys. 2011, 11, 1237–1242. [Google Scholar] [CrossRef]
- Thongpan, W.; Louloudakis, D.; Pooseekheaw, P.; Kumpika, T.; Kantarak, E.; Sroila, W.; Panthawan, A.; Thongsuwan, W.; Singjai, P. Porous CuWO4/WO3 composite films with improved electrochromic properties prepared by sparking method. Mater. Lett. 2019, 257, 126747. [Google Scholar] [CrossRef]
- Ohsaka, T. Temperature Dependence of the Raman Spectrum in Anatase TiO2. J. Phys. Soc. Jpn. 1980, 48, 1661–1668. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cheong, H.; Seong, M.J.; Liu, P.; Tracy, C.; Mascarenhas, A.; Pitts, J.; Deb, S.K. Raman spectroscopic studies of amorphous vanadium oxide thin films. Solid State Ionics 2003, 165, 111–116. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Sadhukhan, P.; Chowdhury, S.R.; Maji, P.; Bhattachrya, S.K.; Das, S.; Bhattacharya, S.K. Electrochemical properties of TiO2-V2O5 nanocomposites as a high-performance supercapacitor’s electrode material. Appl. Surf. Sci. 2018, 443, 581–591. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, J.; Wang, Y.; Yu, M.; Zhu, C.; Lan, W.; Liu, X. Effect of the morphology of V2O5/TiO2 nanoheterostructures on the visible light photocatalytic activity. J. Phys. Chem. Solids 2013, 74, 1475–1481. [Google Scholar] [CrossRef]
- Xie, L.; Liu, P.; Zheng, Z.; Weng, S.; Huang, J. Morphology engineering of V2O5/TiO2 nanocomposites with enhanced visible light-driven photofunctions for arsenic removal. Appl. Catal. B Environ. 2016, 184, 347–354. [Google Scholar] [CrossRef]
- Cha, W.; Chin, S.; Park, E.; Yun, S.-T.; Jurng, J. Photocatalytic performance of V2O5/TiO2 materials prepared by chemical vapor condensation and impregnation method under visible-light. Powder Technol. 2014, 258, 352–357. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Godiksen, A.; Poreddy, R.; Mossin, S.; Jensen, A.D.; Fehrmann, R. Promoted V2O5/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2016, 183, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, M.; Sham, T. X-ray photoelectron spectroscopy (XPS) studies of hydrogen reduced rutile (TiO2-x) surfaces. Chem. Phys. Lett. 1982, 92, 670–674. [Google Scholar] [CrossRef]
- Sotoudeh, M.; Abbasnejad, M.; Mohammadizadeh, M.R. First principles study of hydrogen doping in anatase TiO2. Eur. Phys. J. Appl. Phys. 2014, 67, 30401. [Google Scholar] [CrossRef]
- Qin, H.; Wang, K.; Jiang, L.; Li, J.; Wu, X.; Zhang, G. Ultrasonic-assisted fabrication of a direct Z-scheme BiOI/Bi2O4 heterojunction with superior visible light-responsive photocatalytic performance. J. Alloys Compd. 2020, 821, 153417. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Adepu, A.K.; Goskula, S.; Chirra, S.; Siliveri, S.; Gujjula, S.R.; Narayanan, V. Synthesis of a high-surface area V2O5/TiO2–SiO2 catalyst and its application in the visible light photocatalytic degradation of methylene blue. RSC Adv. 2019, 9, 24368–24376. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Yang, M.Q.; Liu, S.; Sun, Y.; Xu, Y.J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, F. Modulation of Interfacial Charge Transfer by Self-assembly of Single-layer Graphene Enwrapped One-dimensional Semiconductors Toward Photoredox Catalysis. J. Mater. Chem. A 2017, 5, 23681. [Google Scholar] [CrossRef]
- Tsang, C.; Manthiram, A. Synthesis of Nanocrystafline V02 and Its Electrochemical Behavior in Lithium Balferies. J. Electrochem. Soc. 1997, 2, 144. [Google Scholar]
- Won, D.-J.; Wang, C.-H.; Jang, H.-K.; Choi, D.-J. Effects of thermally induced anatase-to-rutile phase transition in MOCVD-grown TiO2 films on structural and optical properties. Appl. Phys. A 2001, 73, 595–600. [Google Scholar] [CrossRef]
Sample Availability: Not available. |








| Samples | Atomic Ratio of Ti/V | Particle Size (nm) | Band Gap (eV) | V4+/Vtotal (%) | Degradation Rate (%) |
|---|---|---|---|---|---|
| TiO2 | - | 20 2 | 3.28 | 0 | 52.0 |
| TiV-1 | 4:1 | 35 3 | 3.12 | 14.5 | 53.8 |
| TiV-2 | 2:1 | 42 5 | 2.84 | 22.6 | 60.8 |
| TiV-3 | 1:1 | 48 2 | 2.63 | 24.4 | 64.8 |
| TiV-4 | 1:2 | 55 3 | 2.56 | 18.0 | 60.4 |
| V2O5 | - | 70 4 | 2.32 | 9.1 | 46.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pooseekheaw, P.; Thongpan, W.; Panthawan, A.; Kantarak, E.; Sroila, W.; Singjai, P. Porous V2O5/TiO2 Nanoheterostructure Films with Enhanced Visible-Light Photocatalytic Performance Prepared by the Sparking Method. Molecules 2020, 25, 3327. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153327
Pooseekheaw P, Thongpan W, Panthawan A, Kantarak E, Sroila W, Singjai P. Porous V2O5/TiO2 Nanoheterostructure Films with Enhanced Visible-Light Photocatalytic Performance Prepared by the Sparking Method. Molecules. 2020; 25(15):3327. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153327
Chicago/Turabian StylePooseekheaw, Porntipa, Winai Thongpan, Arisara Panthawan, Ekkapong Kantarak, Wattikon Sroila, and Pisith Singjai. 2020. "Porous V2O5/TiO2 Nanoheterostructure Films with Enhanced Visible-Light Photocatalytic Performance Prepared by the Sparking Method" Molecules 25, no. 15: 3327. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153327