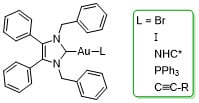
Novel Anticancer NHC*-Gold(I) Complexes Inspired by Lepidiline A
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structural Discussion
2.3. Biological Evaluation
3. Materials and Methods
3.1. General Conditions
3.2. Synthesis
3.2.1. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)gold(I) Bromide (2b)

3.2.2. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)gold(I) Iodide (2c)

3.2.3. 1,3-Dibenzyl-4,5-diphenylimidazolium Hexafluorophosphate (6a)

3.2.4. 1,3-Dibenzyl-4,5-diphenylimidazolium Tetrafluoroborate (6b)

3.2.5. Bis-[1,3-dibenzyl-4,5-diphenylimidazol-2-ylidene]gold(I) Hexafluorophosphate (3a)

3.2.6. Bis-[1,3-dibenzyl-4,5-diphenylimidazol-2-ylidene]gold(I) Tetrafluoroborate (3b)

3.2.7. Triphenylphosphino-(1,3-dibenzyl-4,5-diphenylimidazol-2-ylidene)gold(I) Hexafluorophosphate (4a)

3.2.8. Triphenylphosphino-(1,3-dibenzyl-4,5-diphenylimidazol-2-ylidene)gold(I) Tetrafluoroborate (4b)

3.2.9. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)(ethynyl)gold(I) (5a)

3.2.10. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)(phenylethynyl)gold(I) (5b)

3.2.11. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)(4-methoxyphenylethynyl)gold(I) (5c)

3.2.12. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)(4-fluorophenylethynyl)gold(I) (5d)

3.2.13. (1,3-Dibenzyl-4,5-diphenylimidazol-2-ylidene)[4-(trifluoromethyl)phenylethynyl]gold(I) (5e)

3.3. Structure Determination
3.4. Biological Evaluation
3.4.1. Cell Culture Conditions and Stock Solutions
3.4.2. Anti-Proliferative Activity (MTT-assay)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New Insights in Au-NHCs Complexes as Anticancer Agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maddelein, M.-L.; Wai-Yin Sun, R.; Gornitzka, H.; Cuvillier, O.; Hemmert, C. Pharmacomodulation on Gold-NHC Complexes for Anticancer Applications—Is Lipophilicity the Key Point? Eur. J. Med. Chem. 2018, 157, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lok, C.-N.; Wan, P.-K.; Zhang, Z.-F.; Fung, S.-K.; Che, C.-M. Anticancer Metal-N-Heterocyclic Carbene Complexes of Gold, Platinum and Palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Arcau, J.; Andermark, V.; Rodrigues, M.; Giannicchi, I.; Pérez-Garcia, L.; Ott, I.; Rodríguez, L. Synthesis and Biological Activity of Gold(I) N-Heterocyclic Carbene Complexes with Long Aliphatic Side Chains. Eur. J. Inorg. Chem. 2014, 6117–6125. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V.; Velez, C.; Zayas, B. Imidazolium-Derived Ionic Salts Induce Inhibition of Cancerous Cell Growth through Apoptosis. MedChemComm 2014, 5, 1404–1409. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole Alkaloids from Lepidium Meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent Advances in Gold-NHC Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Meng, G.; Kakalis, L.; Nolan, S.P.; Szostak, M. A Simple 1H NMR Method for Determining the σ-Donor Propertie of N-Heterocyclic Carbenes. Tetrahedron Lett. 2019, 60, 378–381. [Google Scholar] [CrossRef]
- Schmidt, C.; Albrecht, L.; Balasupramaniam, S.; Misgeld, R.; Karge, B.; Brönstrup, M.; Prokop, A.; Baumann, K.; Reichl, S.; Ott, I. A Gold(I) Biscarbene Complex with Improved Activity as a TrxR Inhibitor and Cytotoxic Drug: Comparative Studies with Different Gold Metallodrugs. Metallomics 2019, 11, 533–545. [Google Scholar] [CrossRef]
- Collado, A.; Gómez-Suárez, A.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward Synthesis of [Au(NHC)X] (NHC = N-Heterocyclic Carbene, X = Cl, Br, I) Complexes. Chem. Commun. 2013, 49, 5541–5543. [Google Scholar] [CrossRef]
- Liu, W.; Bensdorf, K.; Proetto, M.; Abram, U.; Hagenbach, A.; Gust, R. NHC Gold Halide Complexes Derived from 4,5-Diarylimidazoles: Synthesis, Structural Analysis, and Pharmacological Investigations as Potential Antitumor Agents. J. Med. Chem. 2011, 54, 8605–8615. [Google Scholar]
- Marzo, T.; Massai, L.; Pratesi, A.; Stefanini, M.; Cirri, D.; Magherini, F.; Becatti, M.; Landini, I.; Nobili, S.; Mini, E.; et al. Replacement of the Thiosugar of Auranofin with Iodide Enhances the Anticancer Potency in a Mouse Model of Ovarian Cancer. ACS Med. Chem. Lett. 2019, 10, 656–660. [Google Scholar] [CrossRef]
- Estrada-Ortiz, N.; Guarra, F.; de Graaf, I.A.M.; Marchetti, L.; de Jager, M.H.; Groothuis, G.M.M.; Gabbiani, C.; Casini, A. Anticancer Gold N-Heterocyclic Carbene Complexes: A Comparative in Vitro and Ex Vivo Study. ChemMedChem 2017, 12, 1429–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberkofler, J.; Aikman, B.; Bonsignore, R.; Pöthig, A.; Platts, J.; Casini, A.; Kühn, F.E. Exploring the Reactivity and Biological Effects of Heteroleptic N-Heterocyclic Carbene Gold (I) -Alkynyl Complexes. Eur. J. Inorg. Chem. 2020, 1040–1051. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Aikman, B.; Döllerer, D.; Klooster, W.T.; Coles, S.J.; Santi, N.; Luk, L.; Casini, A.; Bonsignore, R. Comparative Biological Evaluation and G-Quadruplex Interaction Studies of Two New Families of Organometallic Gold (I) Complexes Featuring N-Heterocyclic Carbene and Alkynyl Ligands. J. Inorg. Biochem. 2020, 202, 110844–110855. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, J.R.; Tomashenko, O.A.; Grachova, E.V.; Starova, G.L.; Sizov, V.V.; Khlebnikov, A.F.; Tunik, S.P. Gold (I)—Alkynyl Complexes with an N-Donor Heterocyclic Ligand: Synthesis and Photophysical Properties. Eur. J. Inorg. Chem. 2017, 4180–4186. [Google Scholar] [CrossRef]
- Andermark, V.; Göke, K.; Kokoschka, M.; Abu el Maaty, M.A.; Lum, C.T.; Zou, T.; Sun, R.W.; Aguiló, E.; Oehninger, L.; Rodríguez, L.; et al. Alkynyl Gold (I) Phosphane Complexes: Evaluation of Structure–Activity-Relationships for the Phosphane Ligands, Effects on Key Signaling Proteins and Preliminary in-Vivo Studies with a Nanoformulated Complex. J. Inorg. Biochem. 2016, 160, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abas, E.; Espallargas, N.; Burbello, G.; Mesonero, J.E.; Rodriguez-Dieguez, A.; Grasa, L.; Laguna, M. Anticancer Activity of Alkynylgold(I) with P(NMe2)3 Phosphane in Mouse Colon Tumors and Human Colon Carcinoma Caco-2 Cell Line. Inorg. Chem. 2019, 58, 15536–15551. [Google Scholar] [CrossRef]
- Walther, W.; Althagafi, D.; Curran, D.; O’Beirne, C.; Mc Carthy, C.; Ott, I.; Basu, U.; Büttner, B.; Sterner-Kock, A.; Müller-Bunz, H.; et al. In-Vitro and in-Vivo Investigations into the Carbene-Gold Anticancer Drug Candidates NHC*-Au-SCSNMe2 and NHC*-Au-S-GLUC against Advanced Prostate Cancer PC3. Anticancer. Drugs 2020, 31, 672–683. [Google Scholar] [CrossRef]
- Hackenberg, F.; Müller-Bunz, H.; Smith, R.; Streciwilk, W.; Zhu, X.; Tacke, M. Novel Ruthenium(II) and Gold(I) NHC Complexes: Synthesis, Characterization, and Evaluation of Their Anticancer Properties. Organometallics 2013, 32, 5551–5560. [Google Scholar] [CrossRef]
- Patil, S.; Deally, A.; Gleeson, B.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Novel Benzyl-Substituted N-Heterocyclic Carbene-Silver Acetate Complexes: Synthesis, Cytotoxicity and Antibacterial Studies. Metallomics 2011, 3, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Muenzner, J.K.; Biersack, B.; Albrecht, A.; Rehm, T.; Lacher, U.; Milius, W.; Casini, A.; Zhang, J.J.; Ott, I.; Brabec, V.; et al. Ferrocenyl-Coupled N-Heterocyclic Carbene Complexes of Gold(I): A Successful Approach to Multinuclear Anticancer Drugs. Chem. Eur. J. 2016, 22, 18953–18962. [Google Scholar] [CrossRef] [PubMed]
- Klauke, K.; Werner, S.; Mohr, F. Ethynyl Complexes of Gold (I) Formed by Transmetallation Using Tin (IV) or Silicon (IV) Compounds. Eur. J. Inorg. Chem. 2018, 1053–1056. [Google Scholar] [CrossRef]
- Heuft, M.A.; Collins, S.K.; Yap, G.P.A.; Fallis, A.G. Synthesis of Diynes and Tetraynes from in Situ Desilylation/Dimerization of Acetylenes. Org. Lett. 2001, 3, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Flörke, U.; Haupt, H.-J.; Jones, P.G. Trifluoromethyl(Triphenylphosphine)Gold(I). Acta Cryst. 1996, 52, 609–611. [Google Scholar] [CrossRef]
- Barnes, N.A.; Brisdon, A.K.; William Brown, F.R.; Cross, W.I.; Crossley, I.R.; Fish, C.; Herbert, C.J.; Pritchard, R.G.; Warren, J.E. Synthesis of Gold(I) Fluoroalkyl and Fluoroalkenyl-Substituted Phosphine Complexes and Factors Affecting Their Crystal Packing. Dalton Trans. 2011, 40, 1743–1750. [Google Scholar] [CrossRef]
- Paolini, J.P. The Bond Order—Bond Length Relationship. J. Comput. Chem. 1990, 11, 1160–1163. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic Interactions as a Subject of Current Research: An up-Date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef]
- Głodek, M.; Pawlędzio, S.; Makal, A.; Plażuk, D. The Impact of Crystal Packing and Aurophilic Interactions on the Luminescence Properties in Polymorphs and Solvate of Aroylacetylide-Gold(I) Complexes. Chem. Eur. J. 2019, 25, 13131–13145. [Google Scholar] [CrossRef]
- Tabrizi, L.; Abyar, F. Conjugation of Gold(III) Complex with Vitamin B1 and Chlorambucil Derivatives: Anticancer Evaluation and Mechanistic Insights. Metallomics 2020, 12, 721–731. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Cryst. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
Sample Availability: Samples of the compounds 2b–5e are not available from the authors. |









| C(8)-Au | Au-X | C(8)-Au-X | C(30)-C(31) | C(31)-C(32) | Au···Au Contact | |
|---|---|---|---|---|---|---|
| 2b a | 2.000(3) | 2.4043(3) | 177.379(8) | - | - | 3.5850(3) |
| 2c b | 2.0057(18) | 2.55545(15) | 174.66(5) | - | - | 3.5449(3) |
| 3a c | 2.022(2) | 2.021(2) | 176.14(8) | - | - | - |
| 3b c | 2.022(3) | 2.019(3) | 173.92(10) | - | - | - |
| 4a d | 2.038(2) | 2.2808(5) | 177.84(6) | - | - | - |
| 4b d | 2.035(2) | 2.2834(6) | 179.40(6) | - | - | - |
| 5a e | 2.018(2) | 1.993(3) | 175.94(9) | 1.198(4) | - | 3.5242(3) |
| 5b e | 2.028(4) | 1.994(4) | 179.65(16) | 1.196(6) | 1.437(5) | - |
| 5c e | 2.019(2) | 1.994(2) | 173.28(9) | 1.197(4) | 1.442(3) | - |
| 5d e | 2.021(4) | 1.982(5) | 173.24(18) | 1.217(7) | 1.430(7) | 3.2077(9) 3.2666(9) |
| 5e e | 2.033(4) | 1.999(4) | 173.46(17) | 1.193(6) | 1.445(6) | 3.3318(5) |
| Compound | HCT-116wt | MCF-7topo | Compound | HCT-116wt | MCF-7topo |
|---|---|---|---|---|---|
| Cisplatin a | 5.42 ± 0.12 | 18.21 ± 0.10 | 4a | 1.3 ± 0.1 | 1.5 ± 0.2 |
| Auranofin a | 3.78 ± 0.10 | 2.89 ± 0.05 | 4b | 2.3 ± 0.2 | 2.4 ± 0.2 |
| 2a | 22.7 ± 1.2 | 20.8 ± 1.9 | 5a | 3.2 ± 0.3 | 7.3 ± 0.8 |
| 2b | 16.7 ± 2.5 | 12.8 ± 2.4 | 5b | 14.1 ± 1.3 | >50 |
| 2c | 0.64 ± 0.01 | 1.1 ± 0.2 | 5c | 8.3 ± 0.5 | >50 |
| 3a | 0.41 ± 0.01 | 0.70 ± 0.06 | 5d | >50 | >50 |
| 3b | 0.29 ± 0.01 | 0.80 ± 0.04 | 5e | >50 | >50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curran, D.; Müller-Bunz, H.; Bär, S.I.; Schobert, R.; Zhu, X.; Tacke, M. Novel Anticancer NHC*-Gold(I) Complexes Inspired by Lepidiline A. Molecules 2020, 25, 3474. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153474
Curran D, Müller-Bunz H, Bär SI, Schobert R, Zhu X, Tacke M. Novel Anticancer NHC*-Gold(I) Complexes Inspired by Lepidiline A. Molecules. 2020; 25(15):3474. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153474
Chicago/Turabian StyleCurran, Danielle, Helge Müller-Bunz, Sofia I. Bär, Rainer Schobert, Xiangming Zhu, and Matthias Tacke. 2020. "Novel Anticancer NHC*-Gold(I) Complexes Inspired by Lepidiline A" Molecules 25, no. 15: 3474. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153474