Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide
Abstract
:1. Introduction
2. Results
2.1. MA Decreases Proliferation of B16F10 Cells by a Dose-Dependent Mechanism
2.2. H2O2 Modifies Cell Viability
2.3. Maslinic Acid’s Influence on Mitochondrial-Membrane Potential
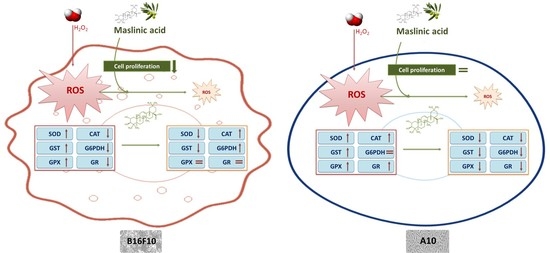
2.4. MA Exerts Antioxidant Activity, Modulating Enzymatic Defense System
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cell Lines and Cultures
4.3. MTT Assay
4.4. Flow-Cytometry Analysis of the Mitochondrial-Membrane Potential
4.5. Antioxidant Enzyme Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez-Rodríguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, J.A.; Adroher, F.J.; Vargas, A.M.; Osuna, A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi. Int. J. Biochem. 1987, 19, 1085–1089. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. I: Citrate synthase, NADP-isocitrate and succinate dehydrogenases. Arch. Biochem. Biophys. 1988, 267, 252–261. [Google Scholar] [CrossRef]
- Adroher, F.J.; Osuna, A.; Lupiáñez, J.A. Differential energetic metabolism during Trypanosoma cruzi differentiation. II. Hexokinase, phosphofructokinase and pyruvate kinase. Mol. Cell. Biochem. 1990, 94, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J.; Barroso, J.B.; de la Higuera, M.; Lupiáñez, J.A. Relationship between growth and protein turnover rates and nucleic acids in the liver of rainbow trout (Oncorhynchus mykiss) during development. Can. J. Fish. Aquat. Sci. 1998, 55, 649–657. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; Aranda, F.; de la Higuera, M.; Lupiáñez, J.A. Selective changes in the protein-turnover rates and nature of growth induced in trout liver by long-term starvation followed by re-feeding. Mol. Cell. Biochem. 1999, 201, 1–10. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary alterations in protein, carbohydrates and fat increase liver protein-turnover rate and decrease overall growth rate in the rainbow trout (Oncorhynchus mykiss). Mol. Cell. Biochem. 2000, 209, 97–104. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Dietary-protein effects on growth and fractional protein-synthesis and degradation rates in liver and white muscle of rainbow-trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 35–46. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragón, J.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Carbohydrate deprivation reduces NADPH-production in fish liver but not in adipose tissue. Int. J. Biochem. Cell Biol. 2001, 33, 785–796. [Google Scholar] [CrossRef]
- Peragón, J.; Barroso, J.B.; García-Salguero, L.; de la Higuera, M.; Lupiáñez, J.A. Growth, protein-turnover rates and nucleic-acid concentrations in the white muscle of rainbow trout during development. Int. J. Biochem. Cell Biol. 2001, 33, 1227–1238. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; García-Rejón, L.; Lupiáñez, J.A.; de la Higuera, M. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout (Oncorhynchus mykiss). II. Adaptive response of glucose 6-phosphate dehydrogenase activity to high-carbohydrate/low-protein and high-fat/on-carbohydrate diets. Aquacult. Nutr. 1996, 2, 193–200. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernández-Marcos, P.J.; Brioche, T.; Gómez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Vina, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, J.P.; Southworth, R.; Medina, R.A.; Davidson, S.M.; Duchen, M.R.; Shattock, M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovas. Res. 2006, 72, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Kurze, A.K.; Buhs, S.; Eggert, D.; Oliveira-Ferrer, L.; Muller, V.; Niendorf, A.; Wagener, C.; Nollau, P. Immature O-glycans recognized by the macrophage glycoreceptor CLEC10A (MGL) are induced by 4-hydroxy-tamoxifen, oxidative stress and DNA-damage in breast cancer cells. Cell Commun. Signal. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic acid, a triterpene from olive, affects the antioxidant and mitochondrial status of B16F10 melanoma cells grown under stressful conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.; Hafeez, Z.B.; Bhat, A.R.; Rizvi, M.A.; Thakur, S.C.; Azam, A.; Athar, F. Antioxidant and apoptotic effects of Callistemon lanceolatus leaves and their compounds against human cancer cells. Biomed. Pharmacother. 2018, 106, 1195–1209. [Google Scholar] [CrossRef]
- De Santiago-Arteche, R. Efecto de la Quimioterapia Antineoplásica en Pacientes con Cancer Colorectal Sobre Biomarcadores del Estrés Oxidativo y del Estado Redox Plasmático. Ph.D. Thesis, University of Burgos, Burgos, Spain, 2010; p. 262. [Google Scholar]
- Zhang, Y.S.; Ning, Z.X.; Yang, S.Z.; Wu, H. Antioxidation properties and mechanism of action of dihydromyricetin from Ampelopsis grossedentata. Acta Pharm. Sin. 2003, 38, 241–244. [Google Scholar]
- Yang, W.F.; Zhao, W.L. Determination of ginsenosides Re, Rb1 in Panax quinquefolius by micellar electrokinetic chromatography. China J. Chin. Mater. Med. 2003, 28, 1135–1137. [Google Scholar]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martín, R.M.; Rufino-Palomares, E.E.; Martín-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16-F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Herrera-Merchán, A.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Anti-cancer and anti-angiogenic properties of various natural pentacyclic tri-terpenoids and some of their chemical derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M. Bioavailability and metabolism of maslinic acid, a natural pentacyclic triterpene. In Recent Advances in Pharmaceutical Sciences VI; Publisher: Kerala, India, 2016; pp. 131–145. [Google Scholar]
- Siewert, B.; Csuk, R. Membrane damaging activity of a maslinic acid analog. Eur. J. Med. Chem. 2014, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [PubMed]
- Barroso, J.B.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. The influence of dietary-protein on the kinetics of NADPH production systems in various tissues of rainbow-trout (Oncorhynchus mykiss). Aquaculture 1994, 124, 47–59. [Google Scholar] [CrossRef]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J. Agric. Food Chem. 2011, 59, 121–130. [Google Scholar] [CrossRef]
- Chen, J.C.; Zhang, G.H.; Zhang, Z.Q.; Qiu, M.H.; Zheng, Y.T.; Yang, L.M.; Yu, K.B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic acid, a natural T¡triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A.; Peragón, J. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. J. Proteom. 2013, 83, 15–25. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, S.K.; Ryu, S.Y. Cytotoxic triterpenes from stem bark of Physocarpus intermedius. Planta Med. 2000, 66, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Pachón-Pena, G.; Lizarraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-García, F.; Dopazo, J.; Lupiáñez, J.A.; Gunther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in Apc(Min/+) mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; García-Salguero, L.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumour activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, D.; Zhang, X.; Shan, L.; Liu, Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Mol. Cell. Biochem. 2014, 392, 281–287. [Google Scholar] [CrossRef]
- Medina, I.; Lois, S.; Lizarraga, D.; Pazos, M.; Tourino, S.; Cascante, M.; Torres, J.L. Functional fatty fish supplemented with grape procyanidins. Antioxidant and proapoptotic properties on colon cell lines. J. Agric. Food Chem. 2006, 54, 3598–3603. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Choi, B.S.; Kim, H.; Lee, H.J.; Sapkota, K.; Park, S.E.; Kim, S.; Kim, S.J. Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson’s disease. Neurochem. Res. 2014, 39, 84–96. [Google Scholar] [CrossRef]
- Rafatian, G.; Khodagholi, F.; Farimani, M.M.; Abraki, S.B.; Gardaneh, M. Increase of autophagy and attenuation of apoptosis by Salvigenin promote survival of SH-SY5Y cells following treatment with H2O2. Mol. Cell. Biochem. 2012, 371, 9–22. [Google Scholar] [CrossRef]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Yang, Z.G.; Li, H.R.; Wang, L.Y.; Li, Y.H.; Lu, S.G.; Wen, X.F.; Wang, J.; Daikonya, A.; Kitanaka, S. Triterpenoids from Hippophae rhamnoides L. and their nitric oxide production-inhibitory and DPPH radical-scavenging activities. Chem. Pharm. Bull. 2007, 55, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, H.N.; Rolfo, M.; Ferraris, A.M.; Gaetani, G.F. Mechanisms of protection of catalase by NADPH—Kinetics and stoichiometry. J. Biol. Chem. 1999, 274, 13908–13914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, A.; Abellán, E.; Arizcun, M.; Cardenete, G.; Morales, A.E.; Hidalgo, M.C. Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish species. Comp. Biochem. Physiol. A 2017, 203, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Z.; Yang, M.; Liu, R.; Wang, W.; Liu, P.; Guo, D. Structural determination of seven new triterpenoids from Kadsura heteroclita by NMR techniques. Magn. Reson. Chem. 2007, 45, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Subbarao, V. Effect of dexamethasone on ciprofibrate-induced cell proliferation and peroxisome proliferation. Fundam. Appl. Toxicol. 1997, 35, 78–83. [Google Scholar] [CrossRef]
- Rothe, G.; Oser, A.; Valet, G. Dihydrorhodamine 123: A new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Die Nat. 1988, 75, 354–355. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Peragón, J.; Aranda, F.; García-Salguero, L.; Corpas, F.J.; Lupiáñez, J.A. Stimulation of rat-kidney hexose-monophosphate shunt dehydrogenase-activity by chronic metabolic-acidosis. Biochem. Int. 1989, 18, 1041–1050. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification by affinity chromatography of yeast glutathione reductase, the enzyme responsible for the NADPH-dependent reduction of the mixed disulfide of coenzyme A and glutathione. Biochim. Biophys. Acta 1977, 484, 268–274. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound maslinic acid are available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.; A. Lupiáñez, J.; Rufino-Palomares, E.E. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules 2020, 25, 4020. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25174020
Mokhtari K, Pérez-Jiménez A, García-Salguero L, A. Lupiáñez J, Rufino-Palomares EE. Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide. Molecules. 2020; 25(17):4020. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25174020
Chicago/Turabian StyleMokhtari, Khalida, Amalia Pérez-Jiménez, Leticia García-Salguero, José A. Lupiáñez, and Eva E. Rufino-Palomares. 2020. "Unveiling the Differential Antioxidant Activity of Maslinic Acid in Murine Melanoma Cells and in Rat Embryonic Healthy Cells Following Treatment with Hydrogen Peroxide" Molecules 25, no. 17: 4020. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25174020