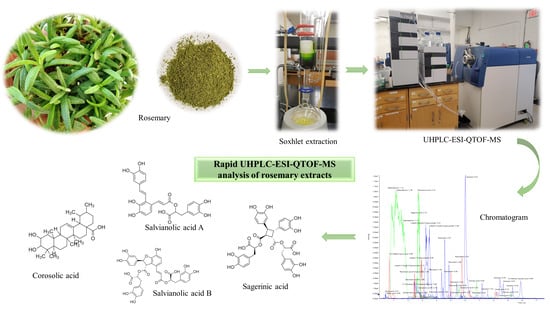
Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantification of Bioactive Compounds by UHPLC-ESI-QTOF-MS
2.2. Identification and Characterization of Bioactive Constituents in R. officinalis
3. Materials and Methods
3.1. Herb Collection
3.2. Chemicals
3.3. Preparation of R. Officinalis Extracts
3.4. UHPLC-ESI-QTOF-MS Method Development
3.5. Identification and Quantification of Polyphenols
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orhan, I.; Aslan, S.; Kartal, M.; Sener, B.; Baser, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Minaiyan, M.; Ghannadi, A.R.; Afsharipour, M.; Mahzouni, P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res. Pharm. Sci. 2011, 6, 13–21. [Google Scholar] [PubMed]
- Al Sereitia, M.R.; Abu-Amerb, K.M.; Sena, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–131. [Google Scholar]
- Jordan, M.J.; Lax, V.; Rota, M.C.; Loran, S.; Sotomayor, J.A. Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Control. 2013, 30, 463–468. [Google Scholar] [CrossRef]
- Duke, J.A. Hand Book of Medical Herbs; CRC Press: Boca Raton, FL, USA, 2001; p. 677. [Google Scholar]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and Phenolic Compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement Altern. Med. 2016. [Google Scholar] [CrossRef] [Green Version]
- Ghisoni, S.; Chiodelli, G.; Rocchetti, G.; Kane, D.; Lucini, L. UHPLC-ESI-QTOF-MS screening of lignans and other phenolics in dry seeds for human consumption. J. Funct. Foods 2017, 34, 229–236. [Google Scholar] [CrossRef]
- Li, H.; Yao, W.; Liu, Q.; Xu, J.; Bao, B.; Shan, M.; Cao, Y.; Cheng, F.; Ding, A.; Zhang, L. Application of UHPLC-ESI-Q-TOF-MS to identify multiple constituents in processed products of the herbal medicine Ligustri Lucidi Fructus. Molecules 2017, 22, 689. [Google Scholar] [CrossRef] [Green Version]
- Sayyad, S.F.; Randive, D.S.; Jagtap, S.M.; Chaudhari, S.R.; Panda, B.P. Preparation and evaluation of fermented Ayurvedic formulation: Arjunarishta. J. Appl. Pharm. Sci. 2012, 5, 122–124. [Google Scholar]
- Valiathan, M.S. The Legacy of Susruta; Orient Longman Pvt Ltd.: Hyderabad, India, 2007. [Google Scholar]
- Sabu, A.; Haridas, M. Fermentation in ancient Ayurveda: Its present implications. Front. Life Sci. 2015, 8, 324–331. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 115, 115–119. [Google Scholar]
- Hussain, M.H. Fast high-performance liquid chromatography and ultraviolet method for determination of phenolic antioxidants in fresh rosemary leaves. J. Nat. Sci. Res. 2015, 5, 89–92. [Google Scholar]
- Borras-Linares, I.; Stojanovic, Z.; Quirantes-Pine, R.; Arraez-Roman, D.; Svarc-Gajic, J.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Tandon, S.; Rane, S. Decoction and Hot Continuous Extraction Techniques. In Extraction Technologies for Medicinal and Aromatic Plants; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; ICS-UNIDO: Trieste, Italy, 2008; pp. 93–106. [Google Scholar]
- Mishra, A.K.; Gupta, A.; Gupta, V.; Sand, R.; Bansal, P. Asava and arishta: An Ayurvedic medicine—An overview. Int. J. Pharm. Biol. Arch. 2010, 1, 24–30. [Google Scholar]
- Mulay, S.; Khale, A. Asavarishtas through improved fermentation technology. Int. J. Pharm. Sci. Res. 2011, 2, 1421–1425. [Google Scholar]
- Ariffin, F.; Heong, C.S.; Bhupinder, K.; Karim, A.A.; Huda, N. Antioxidant capacity and phenolic composition of fermented Centella asiatica herbal teas. J. Sci. Food. Agric. 2011, 91, 2731–2739. [Google Scholar] [CrossRef]
- Heong, C.S.; Bhupinder, K.; Huda, N.; Karim, A.A.; Fazilan, A. Effect of fermentation on the composition of Centella asiatica teas. Am. J. Food Technol. 2011, 6, 581–593. [Google Scholar] [CrossRef] [Green Version]
- Hunaefi, D.; Smetanska, I. The effect of tea fermentation on rosmarinic acid and antioxidant properties using selected in vitro sprout culture of Orthosiphon aristatus as a model study. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound—Assisted extraction of oil from soybeans. Food Res. Int. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.; Gupta, J. Comparison of Different Solvents for Phytochemical Extraction Potential from Datura metel Plant Leaves. Int. J. Biol. Chem. 2017, 11, 17–22. [Google Scholar]
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; McCoy, J.A.; Widrlechner, M.P.; Reddy, M.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Madala, N.E.; Piater, L.; Dubery, I.; Steenkamp, P. Distribution patterns of flavonoids from three Momordica species by ultra-high-performance liquid chromatography quadrupole time of flight mass spectrometry: A metabolomics profiling approach. Rev. Bras. Farmacogn. 2016, 26, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Mercolini, L.; Protti, M.; Saracino, M.A.; Mandrone, M.; Antognoni, F. Analytical profiling of bioactive phenolic compounds in argan (Argania spinosa) leaves by combined microextraction by packed sorbent (MEPS) and LC–DAD-MS/MS. Phytochem. Anal. 2016, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 2, 214–222. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Ri, D.D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Plumb, G.W.; Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Williamson, G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox. Rep. 2002, 7, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londzin, P.; Siudak, S.; Cegieła, U.; Pytlik, M.; Janas, A.; Waligóra, A.; Folwarczna, J. Phloridzin, an apple polyphenol, exerted unfavorable effects on bone and muscle in an experimental model of type 2 diabetes in rats. Nutrients 2018, 10, 1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Son, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of pectolinarigenin and pectolinarin isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.S.; Jan, J.T.; Linag, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [Green Version]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers; ICAR Publications: New Delhi, India, 1985. [Google Scholar]
Sample Availability: Samples of rosemary extracts are available from the authors. |


| Treatment | Polyphenol and Terpenoid Content (µg/g) in Rosemary | |||||
|---|---|---|---|---|---|---|
| Caffeic Acid | Rosmarinic Acid | Luteolon-7-O-Glucoside | Carnosic Acid | Carnosol | Ursolic Acid | |
| T1 | 6.02 ± 0.08 b | 1.51 ± 0.07 a | 1.59 ± 0.22 a | 0.64 ± 0.01 a | 112.06 ± 0.61 b | 5.32 ± 0.17 a |
| T2 | 12.30 ± 0.33 c | 1124.03 ± 13.62 b | 7.14 ± 0.14 bc | 1374.63 ± 7.72 b | 171.52 ± 1.59 b | 6.08 ± 0.17 a |
| T3 | 13.03 ± 0.70 c | 2.51 ± 0.35 a | ND | 0.25 ± 0.01 a | 0.54 ± 0.01 a | 0.36 ± 0.01 a |
| T4 | 38.56 ± 1.58 e | 5428.47 ± 19.69 c | ND | 0.97 ± 0.01 a | 1.91 ± 0.04 a | 2.36 ± 0.07 a |
| T5 | 322.02 ± 3.39 g | 13,310.13 ± 26.12 d | 130.53 ± 5.41 d | 2671.83 ± 20.03 c | 417.21 ± 1.99 c | 89.20 ± 1.92 b |
| T6 | 106.83 ± 1.49 f | 15,242.40 ± 43.62 e | 4.67 ± 0.68 ab | 5.39 ± 0.48 a | 10.82 ± 0.59 a | 6.09 ± 0.19 a |
| T7 | 40.55 ± 0.03 e | 33,491.33 ± 86.29 g | 209.95 ± 8.78 e | 2915.40 ± 33.23 d | 22,000.67 ± 77.39 d | 5144.27 ± 28.68 d |
| T8 | 2.40 ± 0.06 a | 0.26 ± 0.00 a | 0.97 ± 0.01 a | ND | 2.19 ± 0.19 a | 10.37 ± 0.88 a |
| T9 | 23.77 ± 1.63 d | 15,944.00 ± 36.39 f | 9.11 ± 0.35 c | 6.97 ± 0.34 a | 34.98 ± 1.10 a | 1042.88 ± 11.33 c |
| Mean | 62.83 | 9393.85 | 52.99 | 872.01 | 2527.99 | 700.77 |
| F Test | ** | ** | ** | ** | ** | ** |
| SEM ± | 0.85 | 21.02 | 0.81 | 7.61 | 14.90 | 5.95 |
| CD at 1% | 3.46 | 85.56 | 3.28 | 31.00 | 60.67 | 24.21 |
| Sl No | Compound | RT (min) | Mass [M − H]− (m/z) | Formula | Fragments | T1 | T3 |
|---|---|---|---|---|---|---|---|
| 1. | Quinic acid | 0.42 | 191.05681 | C7H12O6 | 85.0297 (42) *, 127.0401 (24), 59.0165 (13) | + | + |
| 2. | Caffeic acid | 0.78 | 179.03520 | C9H8O4 | 135.0438 (100), 134.0370 (21) | + | + |
| 3. | p-Coumaric acid | 1.44 | 163.04014 | C9H8O3 | 119.0500 (100) | + | + |
| 4. | Gallocatechin | 1.65 | 305.07057 | C15H14O7 | 225.1126 (69), 96.9597 (16), 98.9574 (8) | + | + |
| 5. | Luteolin 7-O-rutinoside | 2.18 | 593.15382 | C27H30O15 | 297.0740 (8), 285.0410 (6) | + | − |
| 6. | Salvianolic acid B | 2.29 | 717.14274 | C36H30O16 | 519.0900 (62), 339.0494 (33) | + | − |
| 7. | Rosmarinic acid | 2.58 | 359.07906 | C18H16O8 | 161.0236 (100), 197.0449 (72), 179.0347 (68), 135.0448 (7) | + | + |
| 8. | Isorhamnetin-3-glucoside | 2.80 | 477.10646 | C22H22O12 | 315.0695 (38) | + | − |
| 9. | Apigenin-7-O-glucoside | 2.88 | 431.109907 | C21H20O10 | 269.0449 (100) | + | − |
| 10. | Hesperidin | 2.92 | 609.18546 | C28H34O15 | 301.0695 (100) | + | + |
| 11. | Hispidulin rutinoside | 2.95 | 607.17094 | C28H32O15 | 301.0699 (100), 299.0559 (24) | + | + |
| 12. | Hispidulin-7-O-glucoside | 3.03 | 461.11113 | C22H22O11 | 283.0234 (13), 299.0561(8) | + | − |
| 13. | 6-Hydroxyluteolin-7-O-glucoside | 3.10 | 463.08011 | C21H20O12 | 301.0350 (100) | + | − |
| 14. | Luteolin | 3.11 | 285.03995 | C15H10O6 | 133.0284 (12), 151.0029 (12), 175.0395 (9),199.0395 (8) | − | + |
| 15. | Luteolin-7-O-glucuronide | 3.11 | 461.07495 | C21H18O12 | 285.0385 (100) | + | + |
| 16. | Isorhamnetin | 3.20 | 315.05001 | C16H12O7 | 300.0255 (100), 301.0311 (39) | + | + |
| 17. | Luteolin 3’-acetyl-O-glucuronide isomer I | 3.29 | 503.08570 | C23H20O13 | 285.0381 (100), 443.0587 (100), 381.0606 (35), 399.0720 (28) | + | + |
| 18. | Luteolin 3’-acetyl-O-glucuronide isomer II | 3.38 | 503.08550 | C23H20O13 | 285.0366 (100) | + | + |
| 19. | Apigenin | 3.49 | 269.04559 | C15H10O5 | 117.0356 (12), 149.0356 (8), 225.0560 (5) | − | + |
| 20. | Hesperetin | 3.58 | 301.07074 | C16H14O6 | 242.0571 (87), 284.286.0468 (55), 164.0108(54), 151.0036 (35) | − | + |
| 21. | Diosmetin | 3.58 | 299.05595 | C16H12O6 | 284.0310 (100) | − | + |
| 22. | Luteolin 3’-acetyl-O-glucuronide | 3.62 | 503.08594 | C23H20O13 | 285.4652 (100), 443.0598 (76) | + | + |
| 23. | Rosmanol isomer | 4.07 | 345.16874 | C20H26O5 | 301.1782 (100), 283.1673 (68), 284.1719 (29) | − | + |
| 24. | Pectolinarigenin | 4.15 | 313.07285 | C17H14O6 | 298.0464 (100), 283.0235 (52), 255.0285 (17), 163.0034 (10), 227.0344 (6), 117.0350 (4) | + | + |
| 25. | Rosmanol | 4.30 | 345.17145 | C20H26O5 | 301.1782 (100), 283.1673 (65), 284.1719 (32) | + | + |
| 26. | Genkwanin | 4.58 | 283.06224 | C16H12O5 | 268.0381 (100), 240.0431 (6) | + | + |
| 27. | Rosmanol isomer | 4.60 | 345.17190 | C20H26O5 | 284.1704 (100) | − | + |
| 28. | Rosmadial isomer | 4.98 | 343.15577 | C20H24O5 | 299.1618 (55), 243.1010 (9) | + | + |
| 29. | Rosmanol methyl ether | 5.08 | 359.14801 | C21H18O5 | 315.1577 (19) | − | + |
| 30. | Rosmanol | 5.15 | 345.16890 | C20H26O5 | 283.1669 (69) | − | + |
| 31. | Carnosol isomer | 5.49 | 329.17480 | C20H26O4 | 285.1825 (100) | − | + |
| 32. | Rosmadial | 5.61 | 343.15305 | C20H24O5 | 299.1623 (100) | + | + |
| 33. | Trihydroxy-methoxyflavone | 5.70 | 299.16397 | C16H12O6 | 284.0310 (100) | − | + |
| 34. | Carnosol | 5.75 | 329.17666 | C20H26O4 | 285.1834 (100) | + | + |
| 35. | Carnosic acid | 5.76 | 331.18358 | C20H28O4 | 287.1649 (100) | + | + |
| 36. | Rosmaridiphenol | 6.16 | 315.19780 | C20H26O3 | 285.1843 (19) | + | + |
| 37. | Rosmadial isomer | 6.20 | 343.15249 | C20H24O5 | 299.1598 (56) | + | + |
| 38. | Rosmadial isomer | 6.56 | 343.15233 | C20H24O5 | 299.1602 (68) | + | + |
| 39. | 12-methoxy-carnosic acid | 6.99 | 345.20823 | C21H30O4 | 301.2157 (100), 286.1923 (65) | + | + |
| 40. | Betulinic acid | 8.05 | 455.34934 | C30H48O3 | − | + | − |
| 41. | Ursolic acid | 8.10 | 455.35307 | C30H48O3 | − | + | + |
| Sl No | Compound | RT (min) | Mass [M − H]− (m/z) | Formula | Fragments | T2 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Quinic acid | 0.35 | 191.05670 | C7H12O6 | 85.0301 (39), 93.0354 (18), 127.0406 (15) | + | + | + | + |
| 2. | Syringic acid | 0.42 | 197.04643 | C9H10O5 | 135.0450 (100), 123.0450 (100), 72.9947 (84), 179.0349 (54) | + | + | + | + |
| 3. | Chlorogenic acid | 0.67 | 353.08621 | C16H18O9 | 191.0560 (28) | + | − | + | − |
| 4. | Caffeic acid | 0.78 | 179.03600 | C9H8O4 | 135.0444 (100), 134.0372 (19) | + | + | + | + |
| 5. | 4-O-Caffeoyl quinic acid | 1.00 | 353.08940 | C16H18O9 | 173.0439 (100), 179.0329 (37), 135.0434 (14) | + | − | + | − |
| 6. | p-Coumaric acid | 1.30 | 163.04015 | C9H8O3 | 119.0509 (100) | + | + | + | + |
| 7. | Gallocatechin | 1.40 | 305.07127 | C15H14O7 | 225.1123 (49), 96.9595 (24) | + | + | + | + |
| 8. | 6-Hydroxyluteolin-7-O-glucoside | 1.89 | 463.08849 | C21H20O12 | 286.0427 (100), 301.0350 (69), 285.7613 (44) | + | − | + | − |
| 9. | Luteolin-7-O-glucoside | 2.18 | 447.09508 | C21H20O11 | 285.0413 (53) | + | − | + | + |
| 10. | Luteolin 7-O-rutinoside | 2.18 | 593.15454 | C27H30O15 | 285.0431 (11) | + | + | + | + |
| 11. | Scutellarin | 2.21 | 461.07517 | C21H18O12 | 285.0405 (100), 113.0252 (9), 175.0252 (6) | − | − | − | + |
| 12. | Rosmarinic acid-3-O-glucoside | 2.23 | 521.13273 | C24H26O13 | 359.0792 (100), 324.0832 (78), 323.0785 (60) | + | + | + | + |
| 13. | Salvianolic acid B | 2.29 | 717.15054 | C36H30O16 | 519.0891 (100), 339.0500 (15) | + | + | + | + |
| 14. | Isorhamnetin-3-O-glucoside | 2.33 | 477.10584 | C22H22O12 | 315.0539(38) | + | + | + | + |
| 15. | Sagerinic acid | 2.52 | 719.16630 | C36H32O16 | 359.0761 (100), 179.0336 (20), 161.0223 (16) | + | + | + | + |
| 16. | Rosmarinic acid | 2.56 | 359.07835 | C18H16O8 | 161.0238 (100), 197.0447 (64), 179.0341 (57), 133.0290 (35), 72.9940 (6) | + | + | + | + |
| 17. | Apigenin-7-O-glucoside | 2.59 | 431.09779 | C21H20O10 | 269.0420 (100), 149.0969 (3) | − | − | + | − |
| 18. | Hesperidin | 2.63 | 609.18493 | C28H34O15 | 301.0714 (100) | + | + | + | + |
| 19. | Salvianolic acid A | 2.64 | 493.11382 | C26H22O10 | 295.0615 (100), 185.0224 (43), 109.0289 (11) | + | + | + | + |
| 20. | Diosmin | 2.66 | 607.17017 | C28H32O15 | 301.0704 (100), 299.0551 (59) | + | + | + | + |
| 21. | Hispidulin-7-O-glucoside | 2.68 | 461.11080 | C22H22O11 | 283.0235 (12), 299.0552 (9) | + | − | + | − |
| 22. | Luteolin-7-O-glucuronide | 2.79 | 461.07517 | C21H18O12 | 286.0430 (100), 285.0399 (38) | + | + | + | + |
| 23. | Hesperetin | 2.87 | 301.07245 | C16H14O6 | 286.0459 (12), 164.0100 (4) | − | − | + | − |
| 24. | Methyl rosmarinate | 2.99 | 373.09453 | C19H18O8 | 175.0403 (100), 357.0610 (61), 198.0477 (33), 179.0367 (22), 135.0465 (11) | + | + | + | + |
| 25. | Luteolin 3’-acetyl-O-glucuronide isomer I | 3.10 | 503.08463 | C23H20O1 | 399.0721 (100), 285.7547 (6) | + | + | + | + |
| 26. | Luteolin | 3.11 | 285.04114 | C15H10O6 | 133.0302 (18), 151.0051 (6), 175.0410 (5), 199.0414 (4) | + | + | + | + |
| 27. | Isorhamnetin | 3.17 | 315.05133 | C16H12O7 | 300.0279 (100), 301.0332 (32) | + | + | + | + |
| 28. | Luteolin 3’-acetyl-O-glucuronide isomer II | 3.27 | 503.08550 | C23H20O1 | 286.0415 (100), 285.7547 (62), 443.0607 (60), 399.0721 (7) | + | + | + | + |
| 29. | Apigenin | 3.47 | 269.04675 | C15H10O5 | 117.0350 (8), 151.0043(7), 225.0576 (4) | + | + | + | + |
| 30. | Luteolin 3’-acetyl-O-glucuronide | 3.62 | 503.08530 | C23H20O13 | 286.0415 (100), 443.0607 (47), 285.7547 (38) | + | + | + | + |
| 31. | Diosmetin | 3.64 | 299.05647 | C16H12O6 | 284.0339 (100) | + | + | + | + |
| 32. | Rosmanol isomer | 4.07 | 345.17252 | C20H26O5 | 301.1802 (100), 283.1698 (67) | + | + | + | + |
| 33. | 3,7 Dihydroxy-dimethoxyflavone | 4.15 | 313.07320 | C17H14O6 | 298.0473 (100), 283.0243 (70), 255.0306 (19), 269.0464 (12) | − | − | + | − |
| 34. | Pectolinarigenin | 4.15 | 313.07258 | C17H14O6 | 298.0469 (100), 283.0240 (63) | + | + | − | + |
| 35. | Rosmanol | 4.30 | 345.17100 | C20H26O5 | 283.8834 (18) | + | + | + | + |
| 36. | Pectolinarigenin isomer | 4.37 | 313.07211 | C17H14O6 | 298.0471 (91), 283.0233 (63), 255.0290 (24) | + | − | − | − |
| 37. | Rosmanol isomer | 4.57 | 345.17200 | C20H26O5 | 284.1750 (13), 283.1706 (11) | + | + | + | + |
| 38. | Genkwanin | 4.58 | 283.06218 | C16H12O5 | 268.0398 (79), 240.0434 (5) | + | + | + | + |
| 39. | Rosmadial isomer | 4.80 | 343.15636 | C20H24O5 | 299.1669 (32) | + | + | + | + |
| 40. | Rosmanol isomer | 5.17 | 345.17210 | C20H26O5 | 283.8769 (39) | + | + | + | + |
| 41. | Rosmanol methyl ether | 5.07 | 359.18598 | C21H28O5 | 283.1703 (100), 300.1747 (82) | + | + | & | & |
| 42. | Asiatic acid | 5.57 | 487.34312 | C30H48O5 | & | + | & | & | & |
| 43. | Rosmadial | 5.69 | 343.15590 | C20H24O5 | 299.1645 (9) | + | + | + | + |
| 44. | Trihydroxy- methoxyflavone | 5.69 | 299.16340 | C16H12O6 | 284.0333 (100) | & | + | & | & |
| 45. | Carnosol | 5.75 | 329.17690 | C20H26O4 | 286.1870 (100), 285.1845 (92) | + | + | + | + |
| 46. | Carnosic acid | 5.76 | 331.19253 | C20H28O4 | 287.2007 (100) | + | + | + | + |
| 47. | Rosmadial isomer | 6.04 | 343.17192 | C20H24O5 | 299.1653 (15) | - | + | & | + |
| 48. | Rosmaridiphenol | 6.16 | 315.19689 | C20H28O3 | 284.1860 (4) | + | + | + | + |
| 49. | Carnosol isomer | 6.17 | 329.17560 | C20H26O4 | 286.1880 (100), 285.1852 (58) | + | & | + | & |
| 50. | 12-methoxy-carnosic acid | 6.99 | 345.20853 | C21H30O4 | 286.1943 (100), 301.2186 (81) | + | + | + | + |
| 51. | Micromeric acid | 7.64 | 453.33442 | C30H46O3 | & | + | & | + | & |
| 52. | Betulinic acid | 8.05 | 455.34921 | C30H48O3 | & | + | & | + | & |
| 53. | Ursolic acid | 8.10 | 455.35205 | C30H48O3 | & | + | + | + | + |
| Sl No | Compound | RT (min) | Mass [M − H]− (m/z) | Formula | Fragments | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|
| 1. | Quinic acid | 0.35 | 191.05628 | C7H12O6 | 85.0299 (34), 93.0353 (17), 127.0403 (12) | + | + | + |
| 2. | Syringic acid | 0.42 | 197.04569 | C9H10O5 | 135.0450 (100), 123.0450 (75), 72.9947 (60), 179.0349 (54) | + | - | + |
| 3. | Chlorogenic acid | 0.56 | 353.08506 | C16H18O9 | 191.0545 (26) | & | & | + |
| 4. | Caffeic acid | 0.82 | 179.03589 | C9H8O4 | 135.0441 (100), 134.0370 (23) | + | & | + |
| 5. | 4-O-Caffeoyl quinic acid | 0.85 | 353.08949 | C16H18O9 | 173.0437 (100), 191.0544 (27), 179.0334 (6) | & | & | + |
| 6. | p-Coumaric acid | 1.28 | 163.04014 | C9H8O3 | 119.0509 (100) | + | + | + |
| 7. | Gallocatechin | 1.81 | 305.06932 | C15H14O7 | 96.9588 (65), 225.1109 (59) | + | + | + |
| 8. | 6-Hydroxyluteolin-7-O-glucoside | 1.97 | 463.08518 | C21H20O12 | 301.0350 (69), 285.7613 (44) | & | & | + |
| 9. | 3-p-coumaroylquinic acid | 2.1 | 337.10122 | C16H18O8 | 163.0397 (100), 119.0506 (32) | + | & | & |
| 10. | Luteolin-7-O-glucoside | 2.23 | 447.09286 | C21H20O11 | 285.0377 (29) | + | + | + |
| 11. | Luteolin 7-O-rutinoside | 2.24 | 593.15126 | C27H30O15 | 285.0380 (2) | + | & | + |
| 12. | Rosmarinic acid-3-O-glucoside | 2.25 | 521.12957 | C24H26O13 | 359.0740 (100), 323.0737 (89), 179.0337 (15) | + | & | + |
| 13. | Apigenin-7-O-glucunoride | 2.51 | 445.07602 | C21H18O11 | 269.0437 (100), 113.0255 (10), 175.0252 (9) | & | + | & |
| 14. | Sagerinic acid | 2.52 | 719.15575 | C36H32O16 | 359.0761 (100), 179 (50), 161.0223 (17) | + | & | + |
| 15. | Rosmarinic acid | 2.56 | 359.07651 | C18H16O8 | 161.0229 (100), 197.0434 (78), 179.0330 (60), 133.0285 (15), 72.9934 (8) | + | + | + |
| 16. | Isorhamnetin-3-O-glucoside | 2.56 | 477.10320 | C22H22O12 | 315.0466 (32), 300.0246 (5) | + | & | + |
| 17. | Apigenin-7-O-glucoside | 2.59 | 431.09762 | C21H20O10 | 269.0435 (100) | + | + | + |
| 18. | Isoferulic acid | 2.62 | 193.05102 | C10H10O4 | 134.0386 (89), 133.0295 (75), 178.0271 (12) | + | & | & |
| 19. | Isorhamnetin-3-O-rutinoside | 2.63 | 623.15851 | C28H32O16 | 315.0471 (9) | + | & | + |
| 20. | Hispidulin-7-O-glucuronide | 2.64 | 475.08642 | C22H20O12 | 299.0543 (100), 285.0367 (50), 283.0313 (3) | & | + | & |
| 21. | Hesperidin | 2.64 | 609.18172 | C28H34O15 | 301.0674 (100) | + | & | + |
| 22. | Salvianolic acid A | 2.64 | 493.11132 | C26H22O10 | 295.0615 (100), 185.0224 (43), 109.0289 (11) | + | & | & |
| 23. | Diosmin | 2.66 | 607.16666 | C28H32O15 | 299.0527 (57), 284.0309 (4) | + | + | + |
| 24. | Hispidulin-7-O-glucoside | 2.69 | 461.11138 | C22H22O11 | 298.0477 (18), 283.0234 (7) | + | & | + |
| 25. | Kaempferol-7-O-hexoside | 2.72 | 447.09094 | C21H20O11 | 285.0382 (100) | + | & | + |
| 26. | Luteolin-7-O-glucuronide | 2.83 | 461.06888 | C21H18O12 | 285.7541 (100) | + | + | + |
| 27. | Phlorizin | 2.95 | 435.13210 | C21H24O10 | 273.0767 (100), 167.0349 (28), 125.0247 (5) | + | & | & |
| 28. | Luteolin | 3.12 | 285.04024 | C15H10O6 | 133.0300 (14), 151.0042 (10), 175.0409 (8), 199.0406 (6) | + | + | + |
| 29. | Isorhamnetin | 3.21 | 315.04996 | C16H12O7 | 300.0248 (100), 301.0286 (41) | + | & | + |
| 30. | Luteolin 3’-acetyl-O-glucuronide isomer I | 3.29 | 503.08148 | C23H20O13 | 399.0707(100), 285.4652 (9), 443.0598 (5) | & | + | & |
| 31. | Methyl rosmarinate | 3.30 | 373.08946 | C19H18O8 | 135.0441 (100), 175.0397 (100), 179.0346 (85), 197.0449 (79) | + | & | + |
| 32. | Luteolin 3’-acetyl-O-glucuronide II | 3.38 | 503.08230 | C23H20O13 | 286.0407 (100), 285.4649 (38) | + | + | + |
| 33. | Apigenin | 3.50 | 269.04386 | C15H10O5 | 117.0347 (11), 151.0036 (11), 225.0557 (5) | + | + | + |
| 34. | Salvianolic acid B | 2.29 | 717.14025 | C36H30O16 | 519.0912 (57), 339.0471 (37) | + | & | & |
| 35. | Hesperetin | 3.58 | 301.07242 | C16H14O6 | 164.0114 (11), 286.0827 (5) | & | + | & |
| 36. | Luteolin 3’-acetyl-O-glucuronide | 3.62 | 503.08259 | C23H20O13 | 443.0607 (100), 285.7547 (68) | + | + | + |
| 37. | Diosmetin | 3.64 | 299.05429 | C16H12O6 | 284.0298 (100) | + | + | + |
| 38. | Rosmanol isomer | 4.07 | 345.16839 | C20H26O5 | 301.1773 (100), 283.1668 (65) | + | & | + |
| 39. | Pectolinarigenin | 4.15 | 313.06990 | C17H14O6 | 298.0474 (100), 283.0237 (59), 255.0295 (24), 163.0037 (15), 117.0345 (7) | + | + | + |
| 40. | Rosmanol | 4.30 | 345.16852 | C20H26O5 | 301.1773 (100), 283.1668 (57) | + | & | + |
| 41. | Pectolinarigenin isomer | 4.37 | 313.06892 | C17H14O6 | 298.0471 (91), 283.0233 (63), 255.0290 (24) | + | & | & |
| 42. | Triptolidenol | 4.45 | 375.15421 | C20H24O7 | 331.1526 (13), 244.1082 (9), 313.1430 (7) | & | & | + |
| 43. | Rosmadial isomer | 4.54 | 343.15249 | C20H24O5 | 299.1610 (9) | + | & | + |
| 44. | Genkwanin | 4.58 | 283.06017 | C16H12O5 | 268.0379 (89), 117.0353 (5), 151.0039 (4) | + | + | + |
| 45. | Rosmanol isomer | 4.59 | 345.16789 | C20H26O5 | 284.1687 (40), 283.8801 (23) | + | & | + |
| 46. | Rosmanol isomer | 4.80 | 345.16793 | C20H26O5 | 283.1668 (19) | + | & | + |
| 47. | Asiatic acid | 5.47 | 487.34312 | C30H48O5 | & | + | & | & |
| 48. | Rosmadial | 5.71 | 343.15314 | C20H24O5 | 299.1616 (10) | + | + | + |
| 49. | Rosmanol isomer | 5.71 | 345.16811 | C20H26O5 | 283.1670 (12) | & | + | & |
| 50. | Carnosol | 5.75 | 329.17532 | C20H26O4 | 285.1833 (100) | + | + | + |
| 51. | Epirosmanol methyl ether | 5.85 | 359.18381 | C21H28O5 | 283.1665 (97), 329.1719 (16), 300.1713 (15) | + | & | + |
| 52. | Rosmadial isomer | 6.05 | 343.15233 | C20H24O5 | 299.1621 (11) | + | & | + |
| 53. | Carnosic acid | 6.58 | 331.19123 | C20H28O4 | 287.1982 (100) | + | & | + |
| 54. | Corosolic acid | 6.61 | 471.34212 | C30H48O4 | & | & | & | + |
| 55. | 12-methoxy-carnosic acid | 6.99 | 345.20630 | C21H30O4 | 301.2170 (100), 287.1938 (64) | + | & | + |
| 56. | Micromeric acid | 7.84 | 453.34261 | C30H46O3 | & | + | & | + |
| 57. | Betulinic acid | 8.05 | 455.34934 | C30H48O3 | & | + | & | + |
| 58. | Ursolic acid | 8.10 | 455.35011 | C30H48O3 | & | + | + | + |
| Standard | Purity (%) | Formula | Molecular Weight | LOQ (ng/mL) | Calibration Range (ng/mL) | Calibration Equations | Slope (R2) | |
|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | 98.0 | C9H8O4 | 180.00 | 6.0 | 6–250 | y = 0.00523x + 0.00157 | 0.9993 |
| 2 | Rosmarinic acid | 98.0 | C18H16O8 | 360.31 | 6.0 | 6–250 | y = 0.00374x − 0.00269 | 0.9998 |
| 3 | Luteolin-7-O-glucoside | 98.0 | C21H20O11 | 448.38 | 6.0 | 6–250 | y = 0.00347x − 0.00156 | 0.9994 |
| 4 | Carnosol | 100.0 | C20H26O4 | 330.40 | 6.0 | 6–250 | y = 0.00673x + 0.03215 | 0.9982 |
| 5 | Carnosic acid | 96.0 | C20H28O4 | 332.43 | 24.0 | 24–1000 | y = 9.06876 × 10−5x + 0.00303 | 0.9984 |
| 6 | Ursolic acid | 97.0 | C30H48O3 | 456.70 | 24.0 | 24–1000 | y = 0.00147x − 0.03017 | 0.9938 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules 2020, 25, 4599. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204599
Sharma Y, Velamuri R, Fagan J, Schaefer J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules. 2020; 25(20):4599. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204599
Chicago/Turabian StyleSharma, Yashaswini, Ravikishore Velamuri, John Fagan, and Jim Schaefer. 2020. "Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods" Molecules 25, no. 20: 4599. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204599