Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of T. vulgaris and A. lappa
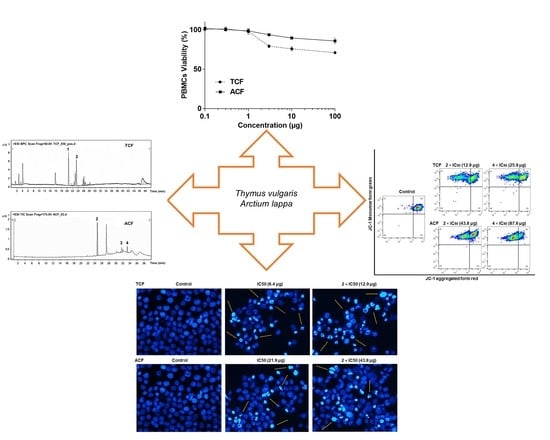
2.2. Apoptosis via Intracellular ROS Generation and MMP Disruption
2.3. Effect of TCF and ACF on Cell Cycle Distribution
2.4. TCF and ACF Affects the Expression of Beclin-1 and LC3B-II
2.5. Phytochemical Study of TCF and ACF
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Plant Extracts
4.3. Cell Culture Conditions
4.4. Resazurin Reduction Assay
4.5. Flow Cytometric Assessment of Apoptosis
4.6. Flow Cytometric Assessment of Mitochondrial Membrane Potential
4.7. Flow Cytometric Assessment Reactive Oxygen Species
4.8. Flow Cytometric Assessment of Cell Cycle Distribution
4.9. Fluorescence Microscopy
4.10. Western Blotting
4.11. LC-ESI/MS Analysis of T. vulgaris and A. lappa Fractions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliusson, G.; Hough, R. Leukemia. Prog. Tumor Res. 2016, 43, 87–100. [Google Scholar] [PubMed]
- Özenver, N.; Dawood, M.; Fleischer, E.; Klinger, A.; Efferth, T. Chemometric and transcriptomic profiling, microtubule disruption and cell death induction by secalonic acid in tumor cells. Molecules 2020, 25, 3224. [Google Scholar] [CrossRef] [PubMed]
- Crowell, J.A. The chemopreventive agent development research program in the division of cancer prevention of the US National Cancer Institute: An overview. Eur. J. Cancer 2005, 41, 1889–1910. [Google Scholar] [CrossRef]
- Maksimović, Z.; Stojanović, D.; Šoštarić, I.; Dajić, Z.; Ristić, M. Composition and radical-scavenging activity of Thymus glabrescens wild (Lamiaceae) essential oil. J. Sci. Food Agric. 2008, 88, 2036–2041. [Google Scholar]
- Ahmed, H.M. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar]
- Grespan, R.; Aguiar, R.P.; Giubilei, F.N.; Fuso, R.R.; Damião, M.J.; Silva, E.L.; Mikcha, J.G.; Hernandes, L.; Bersani Amado, C.; Cuman, R.K.N. Hepatoprotective effect of pretreatment with Thymus vulgaris essential oil in experimental model of acetaminophen-induced injury. Evid. Based Complement. Alternat. Med. 2014, 2014, 954136. [Google Scholar]
- Ayesh, B.M.; Abed, A.A.; Doa’a, M.F. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. Extracts. BMC Res. Notes 2014, 7, 612. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Al-Menhali, A.; Al-Rumaihi, A.; Al-Mohammed, H.; Al-Mazrooey, H.; Al-Shamlan, M.; AlJassim, M.; Al-Korbi, N.; Eid, A.H. Thymus vulgaris (thyme) inhibits proliferation, adhesion, migration, and invasion of human colorectal cancer cells. J. Med. Food 2015, 18, 54–59. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Askari, A.A. Ethnobotany of the Hawraman region of Kurdistan Iraq. Harv. Pap. Bot. 2015, 20, 85–89. [Google Scholar] [CrossRef]
- JianFeng, C.; PengYing, Z.; ChengWei, X.; TaoTao, H.; YunGui, B.; KaoShan, C. Effect of aqueous extract of Arctium lappa L.(burdock) roots on the sexual behavior of male rats. BMC Complement. Altern. Med. 2012, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Badarau, A.S.; Wang, D.; Swamy, M.K.; Shaw, S.; Maggi, F.; Da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium species secondary metabolites chemodiversity and bioactivities. Front. Plant Sci. 2019, 10, 834. [Google Scholar]
- Lou, C.; Zhu, Z.; Zhao, Y.; Zhu, R.; Zhao, H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol. Rep. 2017, 37, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Guo, L.-P.; Hu, X.-L.; Huang, J.; Fan, Y.-H.; Ren, T.-S.; Zhao, Q.-C. Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cell. Mol. Neurobiol. 2015, 35, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Predes, F.S.; Ruiz, A.L.; Carvalho, J.E.; Foglio, M.A.; Dolder, H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement Altern. Med. 2011, 11, 25. [Google Scholar]
- Naqishbandi, A. Plants used in Iraqi traditional medicine in Erbil-Kurdistan region. Zanco J. Med. Sci. 2014, 18, 811–815. [Google Scholar] [CrossRef]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.-F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbaveng, A.T.; Damen, F.; Simo Mpetga, J.D.; Awouafack, M.D.; Tane, P.; Kuete, V.; Efferth, T. Cytotoxicity of crude extract and isolated constituents of the Dichrostachys cinerea bark towards multifactorial drug-resistant cancer cells. Evid. Based Complement. Alternat. Med. 2019, 2019, 8450158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mothana, R.A.; Lindequist, U.; Gruenert, R.; Bednarski, P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern. Med. 2009, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Radovanovic, A. Evaluation of potential cytotoxic effects of herbal extracts. Serbian J. Exp. Clin. Res. 2015, 16, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.G.; Dawson, J.L.; Grant, S.; Shain, K.H.; Dalton, W.S.; Dai, Y.; Meads, M.; Baz, R.; Kauffman, M.; Shacham, S. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J. Hematol. Oncol. 2016, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva-Jr, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, S.; Piccolella, S.; Papale, F.; Nocera, P.; Lettieri, A.; Catauro, M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Al-seragy, I.M.; Kharat, K.R.; Dhabe, A.S. Cell cycle arrest and induction of apoptosis in human breast cancer cells (T-47D) by Annona squamosa L. and Thymus vulgaris L. ethanolic extract. J. Biol. Active Prod. Nat. 2019, 9, 47–56. [Google Scholar] [CrossRef]
- Don, R.A.S.G.; Yap, M.K.K. Arctium lappa L. root extract induces cell death via mitochondrial-mediated caspase-dependent apoptosis in Jurkat human leukemic T cells. Biomed. Pharmacother. 2019, 110, 918–929. [Google Scholar]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Arctigenin induces cell cycle arrest by blocking the phosphorylation of Rb via the modulation of cell cycle regulatory proteins in human gastric cancer cells. Int. Immunopharmacol. 2011, 11, 1573–1577. [Google Scholar] [CrossRef]
- Yan, G.; Elbadawi, M.; Efferth, T. Multiple cell death modalities and their key features. World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Park, J.M.; Tougeron, D.; Huang, S.; Okamoto, K.; Sinicrope, F.A. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS ONE 2014, 9, e100819. [Google Scholar]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2020, 80, 153371. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef]
- Yoshimizu, N.; Otani, Y.; Saikawa, Y.; Kubota, T.; Yoshida, M.; Furukawa, T.; Kumai, K.; Kameyama, K.; Fujii, M.; Yano, M. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment. Pharmacol. Ther. 2004, 20, 95–101. [Google Scholar] [CrossRef]
- Ikeda, A.; Nemoto, K.; Yoshida, C.; Miyata, S.; Mori, J.; Soejima, S.; Yokosuka, A.; Mimaki, Y.; Ohizumi, Y.; Degawa, M. Suppressive effect of nobiletin, a citrus polymethoxyflavonoid that downregulates thioredoxin-interacting protein expression, on tunicamycin-induced apoptosis in SK-N-SH human neuroblastoma cells. Neurosci. Lett. 2013, 549, 135–139. [Google Scholar] [CrossRef]
- Wang, J.-S.; Ren, T.-N.; Xi, T. Ursolic acid induces apoptosis by suppressing the expression of FoxM1 in MCF-7 human breast cancer cells. Med. Oncol. 2012, 29, 10–15. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.-G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch. Pharmacol. Res. 2011, 34, 1363. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, Y.; Zhang, R.; Tu, X.; Gong, X. Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem. Biol. Interact. 2014, 218, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Cmoch, P.; Pakulski, Z.; Swaczynová, J.; Strnad, M. Synthesis of lupane-type saponins bearing mannosyl and 3, 6-branched trimannosyl residues and their evaluation as anticancer agents. Carbohydr. Res. 2008, 343, 995–1003. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Hibasami, H.; Sanpin, K.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by lupeol isolated from mokumen (Gossampinus malabarica L. Merr) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 11, 289–292. [Google Scholar] [CrossRef]
- Redfern, J.; Kinninmonth, M.; Burdass, D.; Verran, J. Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J. Microbiol. Biol. Educ. 2014, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Leich, E.; Weissbach, S.; Klein, H.; Grieb, T.; Pischimarov, J.; Stühmer, T.; Chatterjee, M.; Steinbrunn, T.; Langer, C.; Eilers, M. Multiple myeloma is affected by multiple and heterogeneous somatic mutations in adhesion-and receptor tyrosine kinase signaling molecules. Blood Cancer J. 2013, 3, e102. [Google Scholar] [CrossRef]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.-E.; Volm, M.; Tew, K.D. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [Green Version]
- Özenver, N.; Saeed, M.; Demirezer, L.Ö.; Efferth, T. Aloe-emodin as drug candidate for cancer therapy. Oncotarget 2018, 9, 17770. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.-E.F.; Abdelfatah, S.; Hamed, A.R.; Mohamed, T.A.; Elshamy, A.A.; Saleh, I.A.; Reda, E.H.; Abdel-Azim, N.S.; Shams, K.A.; Sakr, M. Cytotoxicity of 40 Egyptian plant extracts targeting mechanisms of drug-resistant cancer cells. Phytomedicine 2019, 59, 152771. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Bitchagno, G.T.; Kuete, V.; Tane, P.; Efferth, T. Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy. Phytomedicine 2019, 60, 152832. [Google Scholar] [CrossRef]
- Abdelfatah, S.; Böckers, M.; Asensio, M.; Kadioglu, O.; Klinger, A.; Fleischer, E.; Efferth, T. Isopetasin and S-isopetasin as novel P-glycoprotein inhibitors against multidrug-resistant cancer cells. Phytomedicine 2020, 153196. [Google Scholar] [CrossRef]
- Kuete, V.; Mbaveng, A.T.; Sandjo, L.P.; Zeino, M.; Efferth, T. Cytotoxicity and mode of action of a naturally occurring naphthoquinone, 2-acetyl-7-methoxynaphtho [2, 3-b] furan-4, 9-quinone towards multi-factorial drug-resistant cancer cells. Phytomedicine 2017, 33, 62–68. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Fotso, G.W.; Ngnintedo, D.; Kuete, V.; Ngadjui, B.T.; Keumedjio, F.; Andrae-Marobela, K.; Efferth, T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine 2018, 48, 112–119. [Google Scholar] [CrossRef]
- Adem, F.A.; Mbaveng, A.T.; Kuete, V.; Heydenreich, M.; Ndakala, A.; Irungu, B.; Yenesew, A.; Efferth, T. Cytotoxicity of isoflavones and biflavonoids from Ormocarpum kirkii towards multi-factorial drug resistant cancer. Phytomedicine 2019, 58, 152853. [Google Scholar] [CrossRef]
- Nakata, S.; Yoshida, T.; Horinaka, M.; Shiraishi, T.; Wakada, M.; Sakai, T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene 2004, 23, 6261–6271. [Google Scholar] [CrossRef] [Green Version]
- Papi, A.; Farabegoli, F.; Iori, R.; Orlandi, M.; De Nicola, G.R.; Bagatta, M.; Angelino, D.; Gennari, L.; Ninfali, P. Vitexin-2-O-xyloside, raphasatin and (−)-epigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013, 138, 1521–1530. [Google Scholar] [CrossRef]
- Zhao, Q.; Kretschmer, N.; Bauer, R.; Efferth, T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int. J. Cancer 2015, 137, 1446–1456. [Google Scholar] [CrossRef]









| Plants | Solvents | Yields (w/w %) | Color | Consistency |
|---|---|---|---|---|
| T. vulgaris | n-Hexane | 21.172 | Dark green | Greasy, semisolid |
| Chloroform | 2.663 | Dark green | Solid | |
| Ethyl acetate | 28.236 | Pale yellow | Solid | |
| Butanol | 41.507 | Dark red | Gummy | |
| A. lappa | n-Hexane | 1.828 | Yellowish green | Greasy, semisolid |
| Chloroform | 0.454 | Reddish brown | Solid | |
| Ethyl acetate | 4.217 | Reddish brown | Solid | |
| Butanol | 12.756 | Dark red | Gummy |
| T. vulgaris | A. lappa | |||||
|---|---|---|---|---|---|---|
| Fractions | CCRF-CEM | CEM/ADR5000 | CCRF-CEM | CEM/ADR5000 | ||
| IC50 (μg/mL ± SD) | IC50 (μg/mL ± SD) | D.R. | IC50 (μg/mL ± SD) | IC50 (μg/mL ± SD) | D.R. | |
| HF | 31.51 ± 1.63 | 34.02 ± 0.84 | 1.08 | 29.72 ± 1.10 | 55.93 ± 0.68 | 1.88 |
| CF | 2.13 ± 3.77 | 4.00 ± 0.15 | 1.88 | 6.75 ± 0.95 | 14.95 ± 3.28 | 2.21 |
| EF | 4.35 ± 1.18 | 24.85 ± 2.60 | 5.71 | 25.38 ± 3.29 | 29.80 ± 2.32 | 1.17 |
| BF | 28.64 ± 0.35 | 94.35 ± 4.60 | 3.29 | 30.67 ± 2.09 | 93.48 ± 4.89 | 3.05 |
| MM Cell Line | T. vulgaris | A. lappa | ||
|---|---|---|---|---|
| IC50 (µg/mL ± SD) | IC50 (µg/mL ± SD) | |||
| CF | EF | CF | EF | |
| MOLP-8 | 13.45 ± 3.49 | 41.63 ± 0.53 | 39.51 ± 2.30 | 56.92 ± 1.00 |
| NCI-H929 | 6.49 ± 1.48 | 25.55 ±3.78 | 21.9 ± 0.69 | 35.01 ± 0.94 |
| RPMI-8226 | 27.14 ± 0.01 | 30.17 ± 0.17 | 18.26 ± 0.26 | >100 |
| KMS-12BM | 15.28 ± 4.90 | 31.78 ± 3.46 | 22.3 ± 0.18 | 81.2 ± 1.78 |
| KMS-11 | 17.26 ± 2.48 | 35.15 ± 1.93 | 31.95 ± 2.37 | 65.04 ± 1.89 |
| L-363 | 11.28 ± 4.64 | 25.61 ± 2.21 | 46.97 ± 3.66 | 35.62 ± 0.43 |
| JJN-3 | 13.88 ± 1.19 | 30.11 ± 1.69 | 25.99 ± 0.70 | 48.00 ± 4.41 |
| AMO-I | 14.02 ± 2.64 | 35.07 ± 3.23 | 29.11 ± 1.04 | 35.02 ± 0.59 |
| OPM-2 | 6.91 ± 3.70 | 26.06 ± 0.78 | 35.63 ± 4.079 | 45.96 ± 2.49 |
Sample Availability: All samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
N. Adham, A.; F. Hegazy, M.E.; Naqishbandi, A.M.; Efferth, T. Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines. Molecules 2020, 25, 5016. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215016
N. Adham A, F. Hegazy ME, Naqishbandi AM, Efferth T. Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines. Molecules. 2020; 25(21):5016. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215016
Chicago/Turabian StyleN. Adham, Aveen, Mohamed Elamir F. Hegazy, Alaadin M. Naqishbandi, and Thomas Efferth. 2020. "Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines" Molecules 25, no. 21: 5016. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25215016