Efficient Separation of Phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by Polarity-Stepwise Elution Counter-Current Chromatography and Their Antioxidant, Antiglycation, and Aldose Reductase Inhibition Potentials
Abstract
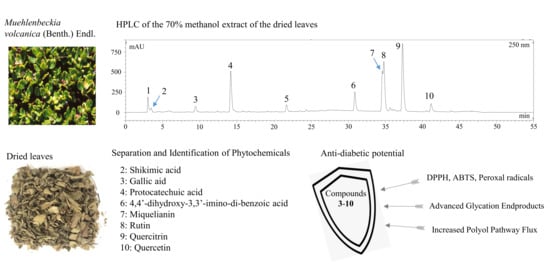
:1. Introduction
2. Results and Discussion
2.1. Antioxidant, Antiglycation, and Aldose Reductase Inhibitory Activities of the 70% Methanol Extract of M. volcanica
2.2. Separation of Compounds by Polarity-Stepwise Elution CCC
2.3. Purification of Compound 2 by Conventional HSCCC and Preparative HPLC
2.4. Purification of Compound 7 by pH-Zone-Refining CCC and Preparative HPLC
2.5. Structure Identification
2.6. The Activity of the Separated Compounds
2.6.1. Antioxidant Activity
2.6.2. Antiglycation Activity
2.6.3. Aldose Reductase Inhibitory Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Plant Extract
3.3. Antioxidant Assay
3.3.1. DPPH Radical Scavenging Assay
3.3.2. ABTS Radical Scavenging Assay
3.3.3. ORAC Assay
3.4. Antiglycation Assay
3.5. Aldose Reductase Inhibition Assay
3.5.1. Preparation of Aldose Reductase
3.5.2. Aldose Reductase Inhibition Assay
3.6. HPLC Analysis
3.7. Separation of Compounds by Polarity-Stepwise Elution CCC
3.7.1. Selection and Preparation of Polarity-Stepwise Elution CCC Solvent Systems
3.7.2. Separation by Polarity-Gradient Elution CCC
3.8. Separation and Purification of Compound 2 by Conventional HSCCC and Preparative HPLC
3.8.1. Separation of Compound 2 by Conventional HSCCC
3.8.2. Purification of Compound 2 by Preparative HPLC
3.9. Separation and Purification of Compound 7 by pH-Zone-Refining CCC and Preparative HPLC
3.9.1. Separation of Compound 7 by pH-Zone-Refining CCC
3.9.2. Purification of Compound 7 by Preparative HPLC
3.10. Structure Elucidation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Maccari, R.; Ottanà, R. Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: New insights and future directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef]
- Elosta, A.; Ghous, T.; Ahmed, N. Natural products as anti-glycation agents: Possible therapeutic potential for diabetic complications. Curr. Diabetes Rev. 2012, 8, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Anim. Models Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.; Van Hinsbergh, V.W. Fructose-mediated non-enzymatic glycation: Sweet coupling or bad modification. Diabetes Metab. Res. Rev. 2004, 20, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Nazarian-Samani, Z.; Sewell, R.D.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal plants with multiple effects on diabetes mellitus and its complications: A systematic review. Curr. Diabetes Rep. 2018, 18, 72. [Google Scholar]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- GBIF. Muehlenbeckia volcanica (Benth.) Endl. Available online: https://www.gbif.org/species/4038573 (accessed on 12 December 2020).
- Paniagua-Zambrana, N.Y.; Bussmann, R.W. Muehlenbeckia volcanica (Benth.) Endl. P olygonaceae. In Ethnobotany of the Andes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–5. [Google Scholar]
- De-la-Cruz, H.; Vilcapoma, G.; Zevallos, P.A. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. J. Ethnopharmacol. 2007, 111, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hwang, S.H.; Quispe, Y.N.G.; Arce, P.H.G.; Lim, S.S. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017, 231, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rosso Zelada, Y.F. Efecto del extracto hidroalcohólico del fruto de Muehlenbeckia volcánica (Benth) Endl. (mullaca) Sobre la Glicemia en Rattus Norvegicusvar. Albinus con Diabetes Inducida. 2019. Available online: http://repositorio.uladech.edu.pe/handle/123456789/14412 (accessed on 12 December 2020).
- La Torre, V.E.E.V.; Ramirez, J.K.; Rodríguez-Silva, C.N.; Velasquez-Arevalo, S. Muehlenbeckia volcanica (Benth.) Endl.: Revisión de una Polygonaceae peruana de interés científico. Ethnobot. Res. Appl. 2020, 19, 1–9. [Google Scholar]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr. A 2006, 1112, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, X.Y.; Pei, D.; Duan, W.D.; Zhang, X.; Sun, X.; Di, D.L. The applicability of high-speed counter current chromatography to the separation of natural antioxidants. J. Chromatogr. A 2020, 1623, 461150. [Google Scholar] [CrossRef]
- Ying, H.; Jiang, H.; Liu, H.; Chen, F.; Du, Q. Ethyl acetate-n-butanol gradient solvent system for high-speed countercurrent chromatography to screen bioactive substances in okra. J. Chromatogr. A 2014, 1359, 117–123. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, P.; Han, R.; Wu, D.; Gao, J.-M.; Wu, S. Methanol linear gradient counter-current chromatography for the separation of natural products: Sinopodophyllum hexandrum as samples. J. Chromatogr. A 2019, 1603, 251–261. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Z.; Guo, X.; Yuan, J.; Zheng, C. Preparative separation of gingerols from Zingiber officinale by high-speed counter-current chromatography using stepwise elution. Food Chem. 2011, 125, 1476–1480. [Google Scholar] [CrossRef]
- Guimarães Leitão, G.; Santos El-Adji, S.; De Melo, W.A.L.; Guimarães Leitão, S.; Brown, L. Separation of free and glycosylated flavonoids from Siparuna guianensis by gradient and isocratic CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2041–2051. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hao, M.; Wu, W.; Zhang, N.; Isaac, A.T.; Yin, J.; Zhu, X.; Du, L.; Yin, X. Antidiabetic cataract effects of GbE, rutin and quercetin are mediated by the inhibition of oxidative stress and polyol pathway. Acta Biochim. Pol. 2018, 65, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brent Friesen, J.; Pauli, G.F. GUESS—A generally useful estimate of solvent systems for CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Shehzad, O.; Khan, S.; Ha, I.J.; Park, Y.; Tosun, A.; Kim, Y.S. Application of stepwise gradients in counter-current chromatography: A rapid and economical strategy for the one-step separation of eight coumarins from Seseli resinosum. J. Chromatogr. A 2013, 1310, 66–73. [Google Scholar] [CrossRef]
- Just, J.; Deans, B.J.; Olivier, W.J.; Paull, B.; Bissember, A.C.; Smith, J.A. New method for the rapid extraction of natural products: Efficient isolation of shikimic acid from star anise. Org. Lett. 2015, 17, 2428–2430. [Google Scholar] [CrossRef]
- Hernández-García, E.; García, A.; Avalos-Alanís, F.G.; Rivas-Galindo, V.M.; Delgadillo-Puga, C.; Del Rayo Camacho-Corona, M. Nuclear magnetic resonance spectroscopy data of isolated compounds from Acacia farnesiana (L.) Willd fruits and two esterified derivatives. Data Brief 2019, 22, 255–268. [Google Scholar] [CrossRef]
- Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3, 4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59. [Google Scholar]
- Klika, K.D.; Khallouki, F.; Owen, R.W. Amino phenolics from the fruit of the argan tree Argania spinosa (Skeels L.). Z. Naturforsch. 2014, 69, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.H.; Jensen, A.G.; Cornett, C.; Bjørnsdottir, I.; Taylor, S.; Wright, B.; Wilson, I.D. High-performance liquid chromatography on-line coupled to high-field NMR and mass spectrometry for structure elucidation of constituents of Hypericum perforatum L. Anal. Chem. 1999, 71, 5235–5241. [Google Scholar] [CrossRef]
- Napolitano, J.G.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Complete 1H NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magn. Reson. Chem. 2012, 50, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, S.S.; Bekbolatova, E.; Cotrim, M.D.; Sakipova, Z.; Ibragimova, L.; Kukula-Koch, W.; Giorno, T.B.S.; Fernandes, P.D.; Fonseca, D.A.; Boylan, F. Chemistry and pharmacology of the Kazakh Crataegus almaatensis pojark: An Asian herbal medicine. Antioxidants 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a structural and analytical tool of intra-and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Yigitturk, G.; Acara, A.C.; Erbas, O.; Oltulu, F.; Yavasoglu, N.U.K.; Uysal, A.; Yavasoglu, A. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed. Pharmacother. 2017, 87, 240–246. [Google Scholar] [CrossRef]
- Adedara, I.A.; Okpara, E.S.; Busari, E.O.; Omole, O.; Owumi, S.E.; Farombi, E.O. Dietary protocatechuic acid abrogates male reproductive dysfunction in streptozotocin-induced diabetic rats via suppression of oxidative damage, inflammation and caspase-3 activity. Eur. J. Pharmacol. 2019, 849, 30–42. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell. Biochem. 2006, 293, 211. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Rao, U.M.; Pandian, M.R. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: Induced diabetic rat tissues. Mol. Cell. Biochem. 2011, 358, 121. [Google Scholar] [CrossRef]
- Mahesh, T.; Menon, V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, I.S.; Lee, Y.M.; Lee, Y.; Kim, J.H.; Kim, J.S. 2′′, 4′′-O-diacetylquercitrin, a novel advanced glycation end-product formation and aldose reductase inhibitor from Melastoma sanguineum. Chem. Pharm. Bull. 2013, 61, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.R.; Shim, S.M. Inhibitory effect of polyphenols in Houttuynia cordata on advanced glycation end-products (AGEs) by trapping methylglyoxal. LWT Food Sci. Technol. 2015, 61, 158–163. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Sena, C.; Seiça, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath, C.; Sang, S.; Ahmedna, M. In vitro and in vivo inhibition of aldose reductase and advanced glycation end products by phloretin, epigallocatechin 3-gallate and [6]-gingerol. Biomed. Pharmacother. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Muthenna, P.; Akileshwari, C.; Saraswat, M.; Petrash, J.M. Inhibition of aldose reductase and sorbitol accumulation by dietary rutin. Curr. Sci. 2011, 1191–1197. [Google Scholar]
- Varma, S.; Mizuno, A.; Kinoshita, J. Diabetic cataracts and flavonoids. Science 1977, 195, 205–206. [Google Scholar] [CrossRef]
- Krishna, K.M.; Annapurna, A.; Gopal, G.S.; Chalam, C.R.; Madan, K.; Kumar, V.K.; Prakash, G.J. Partial reversal by rutin and quercetin of impaired cardiac function in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2005, 83, 343–355. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Yadav, U.C.; Reddy, A.B.; Saxena, A.; Tammali, R.; Shoeb, M.; Ansari, N.H.; Bhatnagar, A.; Petrash, M.J.; Srivastava, S. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011, 191, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Ramana, K.V.; Srivastava, S.K. Aldose reductase: A novel therapeutic target for inflammatory pathologies. Int. J. Biochem. Cell Biol. 2010, 42, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borah, J.C. Shikimic acid: A highly prospective molecule in pharmaceutical industry. Curr. Sci. 2015, 109, 1672–1679. [Google Scholar]
- Guillen Quispe, Y.N.; Hwang, S.H.; Wang, Z.; Zuo, G.; Lim, S.S. Screening in vitro targets related to diabetes in herbal extracts from Peru: Identification of active compounds in Hypericum laricifolium Juss. by offline high-performance liquid chromatography. Int. J. Mol. Sci. 2017, 18, 2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC−fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Z.; Quispe, Y.N.G.; Hwang, S.H.; Kim, H.Y.; Kang, B.G.; Lim, S.S. Products. Target guided isolation of potential tyrosinase inhibitors from Otholobium pubescens (Poir.) JW Grimes by ultrafiltration, high-speed countercurrent chromatography and preparative HPLC. Ind. Crop. Prod. 2019, 134, 195–205. [Google Scholar] [CrossRef]






| Antioxidant Assay | Calibration Curves of DPPH and ABTS Radical Inhibition (%) or Net AUC by Trolox a | TEAC b of the Extract | |||
|---|---|---|---|---|---|
| Linearity Range of Trolox (µg/mL) | Progression | r2 | Concentration (µg/mL) and Radical Scavenging Activity (%) or the Net AUC Value of the Extract | TEAC (µg Trolox/µg Extract) | |
| DPPH | 1.56–25.00 | y = 2.8123x + 2.9182 | 0.9999 | 25.00 (36.53 ± 0.47 c) | 0.48 ± 0.01 |
| ABTS | 0.13–4.17 | y = 23.331x − 1.0452 | 0.9998 | 2.08 (23.48 ± 0.41), 4.17 (44.64 ± 0.67) | 0.49 ± 0.02 |
| ORAC | 0.50–4.00 | y = 20.087x + 19.067 | 0.9939 | 1.00 (40.19 ± 1.10), 2.00 (65.31 ± 1.92) | 1.10 ± 0.07 |
| HSCCC Mobile Phase Composition a | K Values of Compounds 1–10 | Settling Times (s) | Phase Ratio b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| n-Hexane/ethyl acetate 3:5 c, v/v | ≪1 | ≪1 | 0.07 | 0.51 | 0.10 | 0.71 | ≪1 | ≪1 | 0.16 | 12.21 | 25 | 0.95 |
| n-Hexane/ethyl acetate 2:5 d, v/v | ≪1 | ≪1 | 0.15 | 0.88 | 0.29 | 1.49 | 0.03 | 0.03 | 0.41 | 15.59 | 18 | 1.03 |
| n-Hexane/ethyl acetate 1:5 e, v/v | ≪1 | ≪1 | 0.38 | 1.66 | 0.86 | 3.18 | 0.09 | 0.08 | 1.13 | 21.81 | 16 | 0.91 |
| Ethyl acetate f | ≪1 | ≪1 | 1.38 | 4.14 | 2.82 | 7.91 | 0.46 | 0.43 | 3.97 | ≫1 | 8 | 1.00 |
| Ethyl acetate/n-butanol 6:1, v/v | 0.10 | 0.12 | 2.05 | 5.29 | 3.49 | 10.74 | 0.80 | 1.04 | 7.43 | ≫1 | 13 | 1.03 |
| Ethyl acetate/n-butanol 5:1, v/v | 0.18 | 0.14 | 2.08 | 5.19 | 3.50 | 12.26 | 0.90 | 1.16 | 7.23 | ≫1 | 14 | 1.05 |
| Ethyl acetate/n-butanol g 4:1 f, v/v | 0.20 | 0.09 | 1.76 | 4.85 | 3.04 | 9.82 | 0.80 | 1.17 | 7.19 | ≫1 | 17 | 1.11 |
| Solvent System | Kupper/lower | αk1/k2 | |
|---|---|---|---|
| 1 | 2 | ||
| n-Butnaol a/(Methanol/Water 1:5, v/v) 1:1, v/v | 0.39 | 0.40 | 0.98 |
| n-Butnaol/(Methanol/Water 1:5, v/v) 1:1, v/v + 26 mM TFA b | 0.68 | 0.53 | 1.29 |
| n-Butnaol/Water 1:1, v/v | 0.13 | 0.28 | 2.16 |
| n-Butnaol/Water 1:1, v/v + 26 mM TFA | 0.59 | 0.39 | 1.52 |
| Solvent System | Kupper/lower | Compound 7 | Compound 8 |
|---|---|---|---|
| (Ethyl acetate/n-butanol a 4:1, v/v)/(methanol/water 1:5, v/v) 1:1, v/v | Kacidb | 6.66 | 1.49 |
| Kbasec | 0.02 | 0.04 | |
| n-Butanol/(methanol/water 1:5, v/v) 1:1, v/v | Kacid | 5.71 | 3.38 |
| Kbase | 0.10 | 0.21 | |
| n-Butanol/water 1:1, v/v | Kacid | 15.80 | 6.51 |
| Kbase | 0.03 | 0.07 |
| Sample | TEAC 1 (µM trolox/µM Compound) | Antiglycation Activity (%) | AR inhibition Activity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | ORAC | Con. 2 (µM) | Inhibition (%) | IC50 3 (µM) | Con. (µM) | Inhibition (%) | IC50 (µM) | |
| Shikimic acid (2) | - 4 | - | - | 100 | - | - | 50 | 7.36 ± 0.30 | - |
| 50 | - | 25 | 5.86 ± 5.42 | ||||||
| 25 | - | 12.5 | 0.32 ± 1.21 | ||||||
| 12.5 | - | 6.25 | - | ||||||
| Gallic acid (3) | 3.01 ± 0.04 a | 3.35 ± 0.06 b | 1.43 ± 0.08 d | 100 | 8.42 ± 1.27 | - | 50 | 13.54 ± 1.21 | - |
| 50 | 3.69 ± 2.97 | 25 | 4.16 ± 1.81 | ||||||
| 25 | - | 12.5 | 4.16 ± 5.43 | ||||||
| 12.5 | - | 6.25 | - | ||||||
| Protocatechuic acid (4) | 0.72 ± 0.01 f | 0.88 ± 0.03 g | 5.79 ± 0.32 b | 100 | 48.41 ± 1.76 | - | 50 | 7.78 ± 1.51 | - |
| 50 | 36.14 ± 0.78 | 25 | 7.14 ± 0.60 | ||||||
| 25 | 11.44 ± 3.92 | 12.5 | 2.67 ± 2.71 | ||||||
| 12.5 | 5.26 ± 4.29 | 6.25 | 0.96 ± 2.11 | ||||||
| 4,4′-dihydroxy-3,3′-imino-di-benzoic acid (6) | 1.38 ± 0.05 d | 1.27 ± 0.03 c | 5.75 ± 0.26 b | 100 | 68.39 ± 0.92 | 60.46 ± 2.62 b | 50 | 53.28 ± 2.76 | 47.03 ± 2.28 a |
| 50 | 44.48 ± 2.54 | 25 | 27.16 ± 0.36 | ||||||
| 25 | 14.95 ± 3.87 | 12.5 | 14.44 ± 0.60 | ||||||
| 12.5 | - | 6.25 | 8.57 ± 0.06 | ||||||
| Miquelianin (7) | 1.97 ± 0.02 c | 0.99 ± 0.05 f | 5.85 ± 0.20 b | 100 | 90.03 ± 1.92 | 24.51 ± 0.52 d | 25 | 76.65 ± 1.21 | 7.93 ± 0.40 c |
| 50 | 77.99 ± 2.28 | 12.5 | 59.13 ± 6.86 | ||||||
| 25 | 49.38 ± 1.26 | 6.25 | 44.46 ± 2.11 | ||||||
| 12.5 | 24.87 ± 2.12 | 3.125 | 29.74 ± 1.21 | ||||||
| Rutin (8) | 0.90 ± 0.09 e | 1.12 ± 0.04 d | 5.77 ± 0.08 b | 100 | 91.51 ± 0.40 | 20.14 ± 0.23 d | 25 | 84.54 ± 1.51 | 3.51 ± 0.12 d |
| 50 | 81.82 ± 0.65 | 12.5 | 75.16 ± 0.90 | ||||||
| 25 | 61.7 ± 2.49 | 6.25 | 62.79 ± 0.30 | ||||||
| 12.5 | 30.78 ± 1.10 | 3.125 | 46.38 ± 1.21 | ||||||
| Quercitrin (9) | 0.92 ± 0.01 e | 1.07 ± 0.05 e | 5.32 ± 0.29 c | 100 | 94.28 ± 1.39 | 17.65 ± 0.28 e | 3.125 | 94.35 ± 0.30 | 0.17 ± 0.00 e |
| 50 | 89.11 ± 1.26 | 0.195 | 54.57 ± 1.78 | ||||||
| 25 | 75.22 ± 2.52 | 0.098 | 33.29 ± 2.17 | ||||||
| 12.5 | 32.3 ± 0.24 | 0.049 | 12.01 ± 0.82 | ||||||
| Quercetin (10) | 2.14 ± 0.03 b | 3.61 ± 0.03 a | 6.93 ± 0.30 a | 100 | 84.31 ± 1.39 | 36.37 ± 0.13 c | 25 | 60.02 ± 1.21 | 16.57 ± 0.14 b |
| 50 | 67.47 ± 0.24 | 12.5 | 42.96 ± 3.02 | ||||||
| 25 | 35.39 ± 0.92 | 6.25 | 26.33 ± 1.21 | ||||||
| 12.5 | 19.43 ± 1.76 | 3.125 | 12.90 ± 2.11 | ||||||
| Aminoguanidine hydrochloride 5 | - | - | - | 4000 | 81.26 ± 0.88 | 2180.90 ± 8.11 a | - | - | - |
| 2000 | 44.58 ± 0.54 | - | - | ||||||
| 1000 | 10.99 ± 0.07 | - | - | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, G.-L.; Kim, H.Y.; Guillen Quispe, Y.N.; Wang, Z.-Q.; Hwang, S.H.; Shin, K.-O.; Lim, S.S. Efficient Separation of Phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by Polarity-Stepwise Elution Counter-Current Chromatography and Their Antioxidant, Antiglycation, and Aldose Reductase Inhibition Potentials. Molecules 2021, 26, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010224
Zuo G-L, Kim HY, Guillen Quispe YN, Wang Z-Q, Hwang SH, Shin K-O, Lim SS. Efficient Separation of Phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by Polarity-Stepwise Elution Counter-Current Chromatography and Their Antioxidant, Antiglycation, and Aldose Reductase Inhibition Potentials. Molecules. 2021; 26(1):224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010224
Chicago/Turabian StyleZuo, Guang-Lei, Hyun Yong Kim, Yanymee N. Guillen Quispe, Zhi-Qiang Wang, Seung Hwan Hwang, Kyong-Oh Shin, and Soon Sung Lim. 2021. "Efficient Separation of Phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by Polarity-Stepwise Elution Counter-Current Chromatography and Their Antioxidant, Antiglycation, and Aldose Reductase Inhibition Potentials" Molecules 26, no. 1: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010224