Human Nails Permeation of an Antifungal Candidate Hydroalcoholic Extract from the Plant Sapindus saponaria L. Rich in Saponins
Abstract
:1. Introduction
2. Results
2.1. Analysis of Phytochemical Contents of ETHOSS
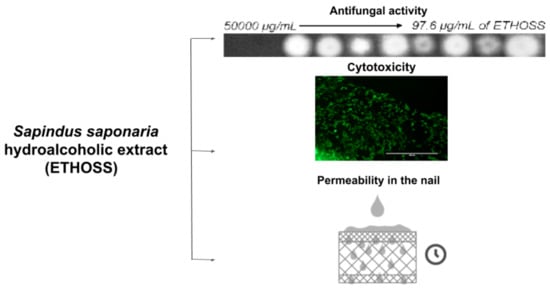
2.2. In Vitro Antifungal Activity of ETHOSS against Pathogens of Onychomycosis
2.3. In Vitro Cytotoxicity Assay
2.4. Ex-Vivo Study on the Permeance of ETHOSS into Human Nails
2.4.1. Spectrometric Characterization of the ETHOSS
2.4.2. Spectrometric Characterization of the Nail before Treatment with the Extract
2.5. Nail Permeation of ETHOSS
3. Discussion
4. Methods and Materials
4.1. Plant Material
4.2. Hydroalcoholic Extract
4.3. Fungi
4.4. Antifungal Activity
4.5. Evaluation of Cytotoxicity of ETHOSS In Vitro Based on Cell Viability
4.6. Analysis of the Permeance of ETHOSS into Human Nails
4.6.1. Human Nails
4.6.2. Photoacoustic Spectroscopy (PAS)
4.6.3. FTIR Coupled to Attenuated Total Reflectance (FTIR-ATR)
4.6.4. Evaluation of ETHOSS on Nail by PAS and FTIR-ATR
4.6.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef]
- Dhamoon, R.K.; Popli, H.; Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Di Chiacchio, N.; Suarez, M.V.; Madeira, C.L.; Loureiro, W.R. An observational and descriptive study of the epidemiology of and therapeutic approach to onychomycosis in dermatology offices in Brazil. An. Bras. Dermatol. 2013, 88 (Suppl. S1), 3–11. [Google Scholar] [PubMed]
- Bodman, M.A.; Krishnamurthy, K. Onychomycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Gupta, A.K.; Gupta, G.; Jain, H.C.; Lynde, C.W.; Foley, K.A.; Daigle, D.; Cooper, E.A.; Summerbell, R.C. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians’ offices. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; Baran, R. The prevalence of onychomycosis in the global population—A literature study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Targhotra, M.; Sahoo, P.K.; Chauhan, M.K. Onychomycosis: Novel strategies for treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101774. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. A Practical Guide to Curing Onychomycosis: How to Maximize Cure at the Patient, Organism, Treatment, and Environmental Level. Am. J. Clin. Dermatol. 2019, 20, 123–133. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Alessandrini, A. Onychomycosis: A Review. J Fungi 2015, 1, 30–43. [Google Scholar] [CrossRef]
- Murgu, M.; Rodrigues-Filho, E. Dereplication of glycosides from Sapindus saponaria using liquid chromatography-mass spectrometry. J. Braz. Chem. Soc. 2006, 17, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Negri, M.; Salci, T.P.; Shinobu-Mesquita, C.S.; Capoci, I.R.G.; Svidzinski, T.I.E.; Kioshima, E.S. Early state research on antifungal natural products. Molecules 2014, 19, 2925–2956. [Google Scholar] [CrossRef]
- Damke, E.; Tsuzuki, J.K.; Cortez, D.A.G.; Ferreira, I.C.P.; Bertoni, T.A.; Batista, M.R.; Donati, L.; Svidzinski, T.I.E.; Consolaro, M.E.L. In vivo activity of Sapindus saponaria against azole-susceptible and resistant human vaginal Candida species. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, S.; Jaigirdar, Q.H.; Islam, M.A.; Azad, A.K. Frequency of Fungal Species of Onychomycosis between Diabetic and Non-Diabetic Patients. Mymensingh. Med. J. 2018, 27, 752–756. [Google Scholar]
- Veiga, F.F.; Gadelha, M.C.; da Silva, M.R.T.; Costa, M.I.; Kischkel, B.; de Castro-Hoshino, L.V.; Sato, F.; Baesso, M.L.; Voidaleski, M.F.; Vasconcellos-Pontello, V.; et al. Propolis Extract for Onychomycosis Topical Treatment: From Bench to Clinic. Front. Microbiol. 2018, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Bombassaro, A.; Vicente, V.A.; Mendes, V.; Baesso, M.L.; Negri, M.; Svidzinski, T.I. Fusarium oxysporum is an onychomycosis etiopathogenic agent. Future Microbiol. 2018, 13, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Shinobu-Mesquita, C.S.; Bonfim-Mendonça, P.S.; Moreira, A.L.; Ferreira, I.C.P.; Donatti, L.; Fiorini, A.; Svidzinski, T.I.E. Cellular Structural Changes in Candida albicans Caused by the Hydroalcoholic Extract from Sapindus saponaria L. Molecules 2015, 20, 9405–9418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Capoci, I.R.G.; Bonfim-Mendonça, P.d.; Arita, G.S.; Pereira, R.R.d.; Consolaro, M.E.L.; Bruschi, M.L.; Negri, M.; Svidzinski, T.I.E. Propolis Is an Efficient Fungicide and Inhibitor of Biofilm Production by Vaginal Candida albicans. Evid. Based Complement. Alternat. Med. 2015, 2015, 287693. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candidaactivity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Terças, A.G.; Monteiro, A.d.; Moffa, E.B.; Santos, J.R.A.d.; de Sousa, E.M.; Pinto, A.R.B.; Costa, P.C.d.; Borges, A.C.R.; Torres, L.M.B.; Barros, A.K.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their antifungal Activities against Candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.-W.; Ng, P.-Y.; Chieng, N.; Ng, S.-F. Moringa oleifera leaf extract–loaded phytophospholipid complex for potential application as wound dressing. J. Drug Deliv. Sci. Technol. 2019, 54, 101329. [Google Scholar] [CrossRef]
- Suresh, C.; Harinath, Y.; Sreenu, B.; Seshaiah, K.; Reddy, A.V.R. Utilization of Sapindus saponaria (soap nut) bark powder for the removal of Cu(II) ions from aqueous environment. Desalination Water Treat. 2016, 57, 16138–16149. [Google Scholar] [CrossRef]
- Cutrín-Gómez, E.; Anguiano-Igea, S.; Delgado-Charro, M.B.; Gómez-Amoza, J.L.; Otero-Espinar, F.J. Effect of Penetration Enhancers on Drug Nail Permeability from Cyclodextrin/Poloxamer-Soluble Polypseudorotaxane-Based Nail Lacquers. Pharmaceutics 2018, 10, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, W.J.; Jones, S.A.; Traynor, M.J.; Guesné, S.; Murdan, S.; Brown, M.B. An investigation of how fungal infection influences drug penetration through onychomycosis patient’s nail plates. Eur. J. Pharm. Biopharm. 2016, 102, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Smułek, W.; Zdarta, A.; Pacholak, A.; Zgoła-Grześkowiak, A.; Marczak, Ł.; Jarzębski, M.; Kaczorek, E. Saponaria officinalis L. extract: Surface active properties and impact on environmental bacterial strains. Colloids Surf. B Biointerfaces 2017, 150, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Ames, F.Q.; Sato, F.; de Castro, L.V.; de Arruda, L.L.M.; da Rocha, B.A.; Cuman, R.K.N.; Baesso, M.L.; Bersani-Amado, C.A. Evidence of anti-inflammatory effect and percutaneous penetration of a topically applied fish oil preparation: A photoacoustic spectroscopy study. J. Biomed. Opt. 2017, 22, 55003. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.T.; Steimacher, A.; Bento, A.C.; Neto, A.M.; Baesso, M.L. Thermal characterization in vitro of human nail: Photoacoustic study of the aging process. Photochem. Photobiol. 2007, 83, 1144–1148. [Google Scholar] [CrossRef]







| Fungi | MIC = MFC * of ETHOSS (μg/mL) |
|---|---|
| T. rubrum CMRP 2913 | 781.25 |
| T. mentagrophytes CMRP 2920 | 390.63 |
| T. interdigitale CMRP 2921 | 195.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, V.; Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Baesso, M.L.; Vesco, B.; Cruz, E.; Ferreira, I.C.P.; Negri, M.; Svidzinski, T.I.E. Human Nails Permeation of an Antifungal Candidate Hydroalcoholic Extract from the Plant Sapindus saponaria L. Rich in Saponins. Molecules 2021, 26, 236. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010236
Mendes V, Veiga FF, de Castro-Hoshino LV, Sato F, Baesso ML, Vesco B, Cruz E, Ferreira ICP, Negri M, Svidzinski TIE. Human Nails Permeation of an Antifungal Candidate Hydroalcoholic Extract from the Plant Sapindus saponaria L. Rich in Saponins. Molecules. 2021; 26(1):236. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010236
Chicago/Turabian StyleMendes, Vanessa, Flávia Franco Veiga, Lidiane Vizioli de Castro-Hoshino, Francielle Sato, Mauro Luciano Baesso, Beatriz Vesco, Elton Cruz, Izabel Cristina Piloto Ferreira, Melyssa Negri, and Terezinha Inez Estivalet Svidzinski. 2021. "Human Nails Permeation of an Antifungal Candidate Hydroalcoholic Extract from the Plant Sapindus saponaria L. Rich in Saponins" Molecules 26, no. 1: 236. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010236