Synthesis of Silsesquioxanes with Substituted Triazole Ring Functionalities and Their Coordination Ability †
Abstract
:1. Introduction
2. Results and Discussion
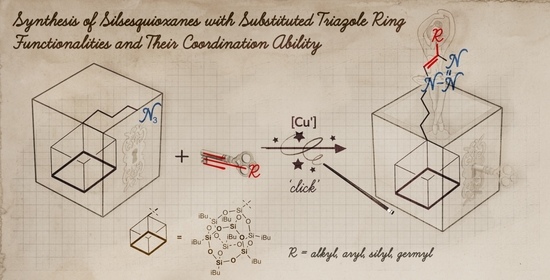
2.1. The Copper(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) Using iBuT8-N3 and DDSQ-2N3
2.2. X-ray Analysis of DDSQ-2A1
2.3. SQs-Based Pyridyl- and Thiophenyl-Triazole Derivatives (iBuT8-A1, DDSQ-2A1, iBuT8-A7) as Bidentate Ligands in the Formation of Coordination Complexes with Selected Transition Metals (TM = Pd, Pt, Rh)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. General Procedure for Copper(I)-Catalyzed Azide-Alkyne Cycloaddition (CuAAC)
3.3.1. Synthetic Procedure with the Use of CuSO4 as Cu(II) Ion Source
3.3.2. Synthetic Procedure with Use of CuBr as Cu(I) Ion Source
3.4. General Procedure for Using SQs-Based Pyridyl-Triazole- and Thiophenyl-Triazole Derivatives as Ligands in the Formation of Coordination Complexes with Selected Transition Metals (Pd, Pt, Rh)
3.4.1. Procedure for the Synthesis of iBuT8-A7-Pd(N^S), DDSQ-A1-[Pd(N^N)]2, and (iBuT8-A1)2-Rh(N^N)
3.4.2. Procedure for Synthesis of iBuT8-A1-Pt(N^N)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9789048137862. [Google Scholar]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-based Hybrid Materials. Dalt. Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C.S. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- John, Ł. Selected developments and medical applications of organic–inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Ahmed, N.; Fan, H.; Dubois, P.; Zhang, X.; Fahad, S.; Aziz, T.; Wan, J. Nano-engineering and micromolecular science of polysilsesquioxane materials and their emerging applications. J. Mater. Chem. A 2019, 7, 21577–21604. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Hreczycho, G. Highly Efficient Catalytic Route for the Synthesis of Functionalized Silsesquioxanes. Inorg. Chem. 2017, 56, 9337–9342. [Google Scholar] [CrossRef]
- Dudziec, B.; Zak, P.; Marciniec, B. Synthetic routes to silsesquioxane-based systems as photoactive materials and their precursors. Polymers 2019, 11, 504. [Google Scholar] [CrossRef] [Green Version]
- Brick, C.M.; Ouchi, Y.; Chujo, Y.; Laine, R.M. Robust Polyaromatic Octasilsesquioxanes from Polybromophenylsilsesquioxanes, Br x OPS, via Suzuki Coupling. Macromolecules 2005, 38, 4661–4665. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Dutkiewicz, M.; Franczyk, A.; Marciniec, B. A facile approach for the synthesis of novel silsesquioxanes with mixed functional groups. New J. Chem. 2019, 43, 18141–18145. [Google Scholar] [CrossRef]
- Żak, P.; Bołt, M.; Grzelak, M.; Rachuta, K.; Dudziec, B.; Januszewski, R.; Marciniec, B.; Marciniak, B. Synthesis and properties of chromophore-functionalized monovinylsilsesquioxane derivatives. New J. Chem. 2020, 44, 7659–7664. [Google Scholar] [CrossRef]
- Grzelak, M.; Frąckowiak, D.; Januszewski, R.; Marciniec, B. Introduction of organogermyl functionalities to cage silsesquioxanes. Dalt. Trans. 2020, 49, 5055–5063. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, J.; Hreczycho, G. Copper(II) triflate-mediated synthesis of functionalized silsesquioxanes via dehydrogenative coupling of POSS silanols with hydrosilanes. Dalt. Trans. 2019, 48, 6341–6346. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Furgal, J.C.; Goodson, T.; Mizumo, T.; Schwartz, M.; Chou, K.; Laine, R.M. 3-D Molecular Mixtures of Catalytically Functionalized [vinylSiO1.5]10/[vinylSiO1.5]12. Photophysical Characterization of Second Generation Derivatives. Chem. Mater. 2012, 24, 1883–1895. [Google Scholar] [CrossRef]
- Vautravers, N.R.; André, P.; Slawin, A.M.Z.; Cole-Hamilton, D.J. Synthesis and characterization of photoluminescent vinylbiphenyl decorated polyhedral oligomeric silsesquioxanes. Org. Biomol. Chem. 2009, 7, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Żak, P.; Dudziec, B.; Kubicki, M.; Marciniec, B. Silylative Coupling versus Metathesis-Efficient Methods for the Synthesis of Difunctionalized Double-Decker Silsesquioxane Derivatives. Chem. A Eur. J. 2014, 20, 9387–9393. [Google Scholar] [CrossRef] [PubMed]
- Asuncion, M.Z.; Roll, M.F.; Laine, R.M. Octaalkynylsilsesquioxanes, Nano Sea Urchin Molecular Building Blocks for 3-D-Nanostructures. Macromolecules 2008, 41, 8047–8052. [Google Scholar] [CrossRef]
- Araki, H.; Naka, K. Syntheses and properties of dumbbell-shaped POSS derivatives linked by luminescent Pi-conjugated units. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4170–4181. [Google Scholar] [CrossRef]
- Cho, H.J.; Hwang, D.H.; Lee, J.I.J.; Jung, Y.K.; Park, J.H.; Lee, J.I.J.; Lee, S.K.; Shim, H.K. Electroluminescent polyhedral oligomeric silsesquioxane-based nanoparticle. Chem. Mater. 2006, 18, 3780–3787. [Google Scholar] [CrossRef]
- Guan, J.; Arias, J.J.R.; Tomobe, K.; Ansari, R.; Marques, M. de F.V.; Rebane, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; Jungsuttiwong, S.; et al. Unconventional Conjugation via vinylMeSi(O−)2 Siloxane Bridges May Imbue Semiconducting Properties in [vinyl(Me)SiO(PhSiO 1.5 ) 8 OSi(Me)vinyl-Ar] Double-Decker Copolymers. ACS Appl. Polym. Mater. 2020, 2, 3894–3907. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Ma, J.; Ding, S. Transition Metal-Catalyzed Cycloaddition of Azides with Internal Alkynes. Asian J. Org. Chem. 2002. [Google Scholar] [CrossRef]
- Huo, J.; Lin, C.; Liang, J. A brief minireview of poly-triazole: Alkyne and azide substrate selective, metal-catalyst expansion. React. Funct. Polym. 2020, 152, 104531. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Anilkumar, G. Recent advances and applications of Glaser coupling employing greener protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Abe, J.; Wang, X.; Matsushima, T.; Murata, H.; Kawakami, Y. Synthesis, characterization, and OLED application of oligo(p-phenylene ethynylene)s with polyhedral oligomeric silsesquioxanes (POSS) as pendant groups. Tetrahedron 2010, 66, 9348–9355. [Google Scholar] [CrossRef]
- Wang, X.; Ervithayasuporn, V.; Zhang, Y.; Kawakami, Y. Reversible self-assembly of dendrimer based on polyhedral oligomeric silsesquioxanes (POSS). Chem. Commun. 2011, 47, 1282–1284. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.; Zheng, S.; Li, S.; Hu, T.; Tang, A.; Gao, C. Water soluble octa-functionalized POSS: All-click chemistry synthesis and efficient host–guest encapsulation. Chem. Commun. 2014, 50, 8712–8714. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; Lu, C.; Nie, J.; Chen, Z.; Ren, J. POSS supported C2-symmetric bisprolinamide as a recyclable chiral catalyst for asymmetric Aldol reaction. Catal. Commun. 2016, 75, 23–27. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, C.; Yang, G.; Chen, Z.; Nie, J. POSS supported diarylprolinol silyl ether as an efficient and recyclable organocatalyst for asymmetric Michael addition reactions. Catal. Commun. 2015, 62, 34–38. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Guang, S.Y.; Xu, H.Y. A versatile nanobuilding precursor for the effective architecture of well-defined organic/inorganic hybrid via click chemistry. Chin. Chem. Lett. 2012, 23, 1095–1098. [Google Scholar] [CrossRef]
- Pu, Y.J.; Yuan, H.; Yang, M.; He, B.; Gu, Z.W. Synthesis of peptide dendrimers with polyhedral oligomeric silsesquioxane cores via click chemistry. Chin. Chem. Lett. 2013, 24, 917–920. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Simple and high yield access to octafunctional azido, amine and urea group bearing cubic spherosilicates. Dalt. Trans. 2017, 46, 221–226. [Google Scholar] [CrossRef]
- Ak, M.; Gacal, B.; Kiskan, B.; Yagci, Y.; Toppare, L. Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages. Polymer (Guildf). 2008, 49, 2202–2210. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Wang, X.; Gacal, B.; Gacal, B.N.; Yagci, Y.; Kawakami, Y. Formation of trimethylsilylated open-cage oligomeric azidophenylsilsesquioxanes. J. Organomet. Chem. 2011, 696, 2193–2198. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zheng, S. Organic-inorganic copolymers with double-decker silsesquioxane in the main chains by polymerization via click chemistry. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4221–4232. [Google Scholar] [CrossRef]
- Liu, Y.; Kigure, M.; Koizumi, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of Tetrachloro, Tetraiodo, and Tetraazido Double-Decker Siloxanes. Inorg. Chem. 2020, 59, 15478–15486. [Google Scholar] [CrossRef]
- Nowacka, M.; Makowski, T.; Kowalewska, A. Hybrid fluorescent poly(Silsesquioxanes) with amide-and triazole-containing side groups for light harvesting and cation sensing. Materials 2020, 13, 4491. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer (Guildf). 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Trastoy, B.; Lõpez-Arbeloa, Í.; Bañuelos, J.; Costela, Ú.; García-Moreno, I.; Chiara, J.L. Click Assembly of Dye-Functionalized Octasilsesquioxanes for Highly Efficient and Photostable Photonic Systems. Chem. A Eur. J. 2011, 17, 13258–13268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiya, R.; Uemura, Y.; Naito, H.; Naka, K.; Haino, T. Chemical Functionalisation and Photoluminescence of Graphene Quantum Dots. Chem. A Eur. J. 2016, 22, 8198–8206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhu, Y.; Guang, S.; Ke, F.; Xu, H. Facile preparation and investigation of the properties of single molecular POSS-based white-light-emitting hybrid materials using click chemistry. New J. Chem. 2018, 42, 555–563. [Google Scholar] [CrossRef]
- Namvari, M.; Du, L.; Stadler, F.J. Graphene oxide-based silsesquioxane-crosslinked networks-synthesis and rheological behavior. RSC Adv. 2017, 7, 21531–21540. [Google Scholar] [CrossRef] [Green Version]
- Gungor, E.; Bilir, C.; Hizal, G.; Tunca, U. Multiarm Star Polymers with POSS at the Periphery EDA. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4835–4841. [Google Scholar] [CrossRef]
- Arslan, I.; Tasdelen, M.A. POSS-based hybrid thermosets via photoinduced copper-catalyzed azide-alkyne cycloaddition click chemistry. Des. Monomers Polym. 2016, 19, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Li, T.; Xu, R.; Gu, X.; Yu, D.; Wu, Y. Synthesis of PS-g-POSS hybrid graft copolymer by click coupling via “graft onto” strategy. J. Appl. Polym. Sci. 2013, 129, 1833–1844. [Google Scholar] [CrossRef]
- Uner, A.; Doganci, E.; Tasdelen, M.A. Non-covalent interactions of pyrene end-labeled star poly(ɛ-caprolactone)s with fullerene. J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Bach, L.G.; Islam, M.R.; Nga, T.T.; Binh, M.T.; Hong, S.S.; Gal, Y.S.; Lim, K.T. Chemical modification of polyhedral oligomeric silsesquioxanes by functional polymer via azide-alkyne click reaction. J. Nanosci. Nanotechnol. 2013, 13, 1970–1973. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Zheng, S. Synthesis of POSS-terminated polycyclooctadiene telechelics via ring-opening metathesis polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 223–233. [Google Scholar] [CrossRef]
- Gauthier, M.; Aridi, T. Synthesis of arborescent polystyrene by “click” grafting. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1730–1740. [Google Scholar] [CrossRef]
- Chang, P.; Xu, S.; Zhao, B.; Zheng, S. A design of shape memory networks of poly(ε-caprolactone)s via POSS-POSS interactions. Polym. Adv. Technol. 2019, 30, 713–725. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Dong, X.H.; Yu, X.; Guo, K.; Su, H.; Yue, K.; Wesdemiotis, C.; Cheng, S.Z.D.; Zhang, W. Bin Giant gemini surfactants based on polystyrene-hydrophilic polyhedral oligomeric silsesquioxane shape amphiphiles: Sequential “click” chemistry and solution self-assembly. Chem. Sci. 2013, 4, 1345–1352. [Google Scholar] [CrossRef]
- Yue, K.; Liu, C.; Guo, K.; Yu, X.; Huang, M.; Li, Y.; Wesdemiotis, C. Sequential “ Click ” Approach to Polyhedral Oligomeric Silsesquioxane-Based Shape Amphiphiles. Macromolecules 2012, 45, 8126–8134. [Google Scholar] [CrossRef]
- Trastoy, B.; Eugenia Pérez-Ojeda, M.; Sastre, R.; Chiara, J.L. Octakis(3-azidopropyl)octasilsesquioxane: A versatile nanobuilding block for the efficient preparation of highly functionalized cube-octameric polyhedral oligosilsesquioxane frameworks through click assembly. Chem. A Eur. J. 2010, 16, 3833–3841. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ojeda, M.E.; Trastoy, B.; Rol, Á.; Chiara, M.D.; García-Moreno, I.; Chiara, J.L. Controlled click-assembly of well-defined hetero-bifunctional cubic silsesquioxanes and their application in targeted bioimaging. Chem. A Eur. J. 2013, 19, 6630–6640. [Google Scholar] [CrossRef] [Green Version]
- Ervithayasuporn, V.; Kwanplod, K.; Boonmak, J.; Youngme, S.; Sangtrirutnugul, P. Homogeneous and heterogeneous catalysts of organopalladium functionalized-polyhedral oligomeric silsesquioxanes for Suzuki-Miyaura reaction. J. Catal. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Wang, X.; Kawakami, Y. Synthesis and characterization of highly pure azido-functionalized polyhedral oligomeric silsesquioxanes (POSS). Chem. Commun. 2009, 60, 5130–5132. [Google Scholar] [CrossRef]
- Vogelsang, D.F.; Dannatt, J.E.; Maleczka, R.E.; Lee, A. Separation of asymmetrically capped double-decker silsesquioxanes mixtures. Polyhedron 2018, 155, 189–193. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-substituted 1H-tetrazoles from nitriles in water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Oliva, A.I.; Pericàs, M.A. Direct copper(I)-catalyzed cycloaddition of organic azides with TMS-protected alkynes. Synlett 2010, 1873–1877. [Google Scholar]
- Demina, M.M.; Nguyen, T.L.H.; Shaglaeva, N.S.; Mareev, A.V.; Medvedeva, A.S. Highly efficient synthesis of 4-trialkylsilyl(germyl)-1H-1,2,3-triazole-5-carbaldehydes. Russ. J. Org. Chem. 2012, 48, 1582–1584. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Hassanzadeh, Z.; Gholamzadeh, P.; Asadi, S.; Badiei, A. Advances in Click Chemistry for the Silica based Material Construction. RSC Adv. 2016, 6, 21979–22006. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Majchrzak, M.; Kubicki, M.; Dudziec, B.; Marciniec, B. The unusual cis- and trans-architecture of dihydrofunctional double-decker shaped silsesquioxane—Design and construction of its ethyl bridged π-conjugated arene derivatives. New J. Chem. 2017, 41, 3290–3296. [Google Scholar] [CrossRef]
- Gavezzotti, A.; Filippini, G. Geometry of the intermolecular X-H⋯Y (X, Y = N, O) hydrogen bond and the calibration of empirical hydrogen-bond potentials. J. Phys. Chem. 1994, 98, 4831–4837. [Google Scholar] [CrossRef]
- Gavezzotti, A. Are crystal structures predictable? Acc. Chem. Res. 1994, 27, 309–314. [Google Scholar] [CrossRef]
- Piec, K.; Kostera, S.; Jędrzkiewicz, D.; Ejfler, J.; John, Ł. Mono-substituted amine-oligosilsesquioxanes as functional tools in Pd(II) coordination chemistry: synthesis and properties. New J. Chem. 2020, 44, 10786–10795. [Google Scholar] [CrossRef]
- Marciniec, B.; Kownacki, I.; Franczyk, A.; Kubicki, M. Silsesquioxyl rhodium(i) complexes—Synthesis, structure and catalytic activity. Dalt. Trans. 2011, 40, 5073–5077. [Google Scholar] [CrossRef]
- Au-Yeung, H.-L.; Leung, S.Y.-L.; Yam, V.W.-W. Supramolecular assemblies of dinuclear alkynylplatinum(II) terpyridine complexes with double-decker silsesquioxane nano-cores: the role of isomerism in constructing nano-structures. Chem. Commun. 2018, 54, 4128–4131. [Google Scholar] [CrossRef]
- Kucuk, A.C.; Matsui, J.; Miyashita, T. Synthesis and photochemical response of Ru(II)-coordinated double-decker silsesquioxane. RSC Adv. 2018, 8, 2148–2156. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.; Loth, M.A.; Payne, M.; Zhang, W.; Smith, J.; Day, C.S.; Parkin, S.R.; Heeney, M.; McCulloch, I.; Anthopoulos, T.D.; et al. High mobility field-effect transistors with versatile processing from a small-molecule organic semiconductor. Adv. Mater. 2013, 25, 4352–4357. [Google Scholar] [CrossRef] [PubMed]
- Dudziec, B.; Rzonsowska, M.; Marciniec, B.; Brząkalski, D.; Woźniak, B. New mono- and diethynylsiloxysilsesquioxanes—Efficient procedures for their synthesis. Dalt. Trans. 2014, 43, 13201–13207. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlisPro v1.171.40.81a; Rigaku Corporation: Oxford, UK, 2020. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sommerfeld, N.S.; Gülzow, J.; Roller, A.; Cseh, K.; Jakupec, M.A.; Grohmann, A.; Galanski, M.; Keppler, B.K. Antiproliferative Copper(II) and Platinum(II) Complexes with Bidentate N,N-Donor Ligands. Eur. J. Inorg. Chem. 2017, 2017, 3115–3124. [Google Scholar] [CrossRef]









 | |||||
 iBuT8-A1 (78%) a |  iBuT8-A2 (90%) a |  iBuT8-A3 (83%) a | |||
 iBuT8-A4 (82%) a |  iBuT8-A5 (90%) a |  iBuT8-A6 (89%) a | |||
 iBuT8-A7 (81%) a |  iBuT8-A8 (84%) b |  iBuT8-A9 (70%) b |  iBuT8-A10 (85%) c | ||
 | |||
 DDSQ-2A1 (85%) a |  DDSQ-2A2 (60%) a |  DDSQ-2A3 (82%) a | |
 DDSQ-2A4 (78%) a |  DDSQ-2A11 (84%) a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzonsowska, M.; Kozakiewicz, K.; Mituła, K.; Duszczak, J.; Kubicki, M.; Dudziec, B. Synthesis of Silsesquioxanes with Substituted Triazole Ring Functionalities and Their Coordination Ability. Molecules 2021, 26, 439. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020439
Rzonsowska M, Kozakiewicz K, Mituła K, Duszczak J, Kubicki M, Dudziec B. Synthesis of Silsesquioxanes with Substituted Triazole Ring Functionalities and Their Coordination Ability. Molecules. 2021; 26(2):439. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020439
Chicago/Turabian StyleRzonsowska, Monika, Katarzyna Kozakiewicz, Katarzyna Mituła, Julia Duszczak, Maciej Kubicki, and Beata Dudziec. 2021. "Synthesis of Silsesquioxanes with Substituted Triazole Ring Functionalities and Their Coordination Ability" Molecules 26, no. 2: 439. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26020439