Root Extracts of Two Cultivars of Paeonia Species: Lipid Composition and Biological Effects on Different Cell Lines: Preliminary Results
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
Extraction Results
2.2. Biological Activity
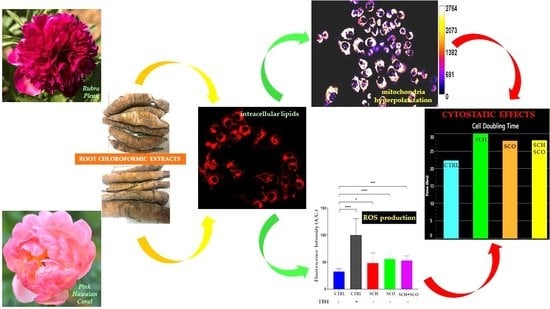
2.2.1. Effects of SCH and SCO on Cell Proliferation
2.2.2. Effects of Chloroformic Extracts on Phenotypic Behavioral Traits of IGROV1
2.2.3. Assessment of Mitochondrial Membrane Potential and Reactive Oxygen Species (ROS) Levels in IGROV1 Treated with Chloroformic Extracts
3. Materials and Methods
3.1. Plant Material, Solvents and Chemicals
3.2. Extraction Procedure
3.3. Cell Culture and Treatments
3.3.1. MTT Assay
3.3.2. Fluorescence Measurements
3.3.3. Confocal Microscopy
3.3.4. Cell Cycle Analysis and Quantitative Phase Imaging (QPI) Microscopy
3.3.5. Cell Viability
3.3.6. Measurement of Intracellular ROS Level
3.3.7. Mitochondrial Membrane Potential Measurement
3.3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wu, S.-H.; Wu, D.-G.; Chen, Y.-W. Chemical Constituents and Bioactivities of Plants from the Genus Paeonia. Chem. Biodiv. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- The American Peony Society: Peony Species. Available online: https://americanpeonysociety.org/wp-content/uploads/2019/03/Peony-Species-Buchite.pdf (accessed on 18 January 2021).
- Kamenetsky, R.; Dole, J. Herbaceous Peony (Paeonia): Genetics, physiology and cut flowers profuction. Floric. Ornam. Biotechnol. 2012, 6, 62–77. [Google Scholar]
- Yan, Z.G.; Xie, L.H.; Wang, N.; Sun, D.Y.; Bai, Z.Z.; Niu, L.X.; Zhang, Y.L.; Ji, X.T. Phenotypic Characteristics and Fatty Acid Composition of Seeds from Different Herbaceous Peony Species Native to China. Chem. Biodivers. 2019, 16, e1800589. [Google Scholar] [CrossRef] [PubMed]
- Halda, J.J.; Waddick, J.W. The Genus Paeonia; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- Fisher, C. Flowers of the Renaissance, 1st ed.; J. Paul Getty Museum: Malibu, CA, USA, 2011. [Google Scholar]
- Ahmad, F.; Tabassum, N.; Rasool, S. Medicinal Uses and Phytoconstituents of Paeonia officinalis. Int. Res. J. Pharm. 2012, 3, 85–87. [Google Scholar]
- Ding, H.-Y.; Lin, H.-C.; Teng, C.-M.; Wu, Y.-C. Phytochemical and pharmacological studies on Chinese Paeonia species. J. Chin. Chem. Soc. 2000, 47, 381–388. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Z.; Peng, Y.; Hou, J.L.; Wei, S.L.; Zhao, Z.Z.; Wang, W.Q. Whole transverse section and specific-tissue analysis of secondary metabolites in seven different grades of root of Paeonia lactiflora using laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 103, 7–16. [Google Scholar] [CrossRef]
- Ko, H.L.; Jung, E.H.; Jung, D.H.; Kim, J.K.; Ku, S.K.; Kim, Y.W.; Kim, S.C.; Zhao, R.; Lee, C.W.; Cho, I.J. Paeonia japonica root extract protects hepatocytes against oxidative stress through inhibition of AMPK-mediated GSK3β. J. Funct. Foods 2016, 20, 303–316. [Google Scholar] [CrossRef]
- Ahmad, F.; Tabassum, N. Preliminary phytochemical, acute oral toxicity and antihepatotoxic study of roots of Paeonia officinalis Linn. Asian Pac. J. Trop. Biomed. 2013, 3, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Ghedira, K.; Goetz, P. Pivoine Paeonia officinalis L. (Paeoniaceae). Phytotherapie 2015, 13, 328–331. [Google Scholar] [CrossRef]
- Marino, G.; Grandi, F.; Muzzi, E.; Giorgioni, M.E. In vitro shoot multiplication and rooting of wild Paeonia officinalis L., subsp. officinalis. Eur. J. Hortic. Sci. 2018, 83, 125–134. [Google Scholar] [CrossRef]
- Babii, F.E.; Babili, M.E.; Fouraste, I.; Chatelain, C. PEONIES: Comparative study by anatomy and TLC of three traditional chinese medicinal plants. Chin. Med. 2013, 4, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Yu, X.; Wu, Y.; Shiraishi, F.; Kawahara, N.; Komatsu, K. Genetic and chemical characterization of white and red peony root derived from Paeonia lactiflora. J. Nat. Med. 2015, 69, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assessment Report on Paeonia Lactiflora Pallas, Radix (Paeoniae Radix Alba) EMA/HMPC/762954/2015. Available online: https://www.ema.europa.eu/en/documents/public-statement/final-public-statement-paeonia-lactiflora-pallas-radix-paeoniae-radix-alba_en.pdf (accessed on 18 January 2021).
- He, C.-N.; Peng, Y.; Zhang, Y.-C.; Xu, L.-J.; Gu, J.; Xiao, P.-G. Phytochemical and biological studies of Paeoniaceae. Chem. Biodiv. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef]
- Manayi, A.; Omidpanah, S.; Barreca, D.; Ficarra, S.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Neuroprotective effects of paeoniflorin in neurodegenerative diseases of the central nervous system. Phytochem. Rev. 2017, 16, 1173–1181. [Google Scholar] [CrossRef]
- Luo, J.; Jin, D.E.; Yang, G.Y.; Zhang, Y.Z.; Wang, J.M.; Kong, W.P.; Tao, Q.W. Total glucosides of paeony for rheumatoid arthritis: A systematic review of randomized controlled trials Complement. Ther. Med. 2017, 34, 46–56. [Google Scholar] [CrossRef]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core beta-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef] [Green Version]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.C.; Liu, Y.Z.; Li, H.X.; Wang, Z. Chebulinic acid and tellimagrandin I inhibit DNA strand breaks by hydroquinone/Cu(II) and H2O2/Cu(II), but potentiate DNA strand breaks by H2O2/Fe(II). Toxicol. In Vitro 2009, 23, 667–673. [Google Scholar] [CrossRef]
- Yi, Z.C.; Liu, Y.Z.; Li, H.X.; Wang, Z. Prooxidant action of chebulinic acid and tellimagrandin I: Causing copper-dependent DNA strand breaks. Toxicol. In Vitro 2009, 23, 425–431. [Google Scholar] [CrossRef]
- Aquino, R.P.; Santoro, A.; Prota, L.; Mencherini, T.; Esposito, E.; Ursini, M.V.; Picerno, P.; Nori, S.; Sansone, F.; Russo, P. Composition and anti-inflammatory activity of extracts from three Paeonia species. Pharmacologyonline 2014, 1, 137–147. [Google Scholar]
- Mencherini, T.; Picerno, P.; Festa, M.; Russo, P.; Capasso, A.; Aquino, R. Triterpenoid Constituents from the Roots of Paeonia rockii ssp. Rockii. J. Nat. Prod. 2011, 74, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-Y.; Chou, T.-H.; Lin, R.-J.; Chan, L.-P.; Wang, G.-H.; Liang, C.-H. Antioxidant and Antimelanogenic Behaviors of Paeonia suffruticosa. Plant Foods Hum. Nutr. 2011, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Tabassum, N. Effect of 70% ethanolic extract of roots of Paeonia officinalis Linn. on hepatotoxicity. J. Acute Med. 2013, 3, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.-Y.; Wu, Y.-C.; Lin, H.-C.; Chan, Y.-Y.; Wu, P.-L.; Wu, T.-S. Glycosides from Paeonia suffrutticosa. Chem. Pharm. Bull. 1999, 47, 652–655. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Han, H.S.; Park, J.H.; Lee, J. Methanol Extract of Peony Root (Peonia lactiflora) and its Ethyl Acetate Fraction Attenuate Heart and Brain Injury in a Rat Model of Ischemia-Reperfusion. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 799–801. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Bernardo, L. The genus Paeonia L. in Italy: Taxonomic survey and revision. Webbia J. Plant Taxon. Geogr. 2004, 59, 215–268. [Google Scholar] [CrossRef]
- Hong, E.; Kim, G.-H. GC-MS Analysis of the Extracts from Korean Cabbage (Brassica campestris L. ssp. pekinensis) and Its Seed. Prev. Nutr. Food Sci. 2013, 18, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, L.H.; Pratiwi, R.; Soesilohadi, R.C.H.; Subin, E.R.; Wahyuni, S.; Jekli; Hartini, Y.S.; Lailaty, I.Q. Repellent activity of Piper spp. leaves extracts on rice ear bugs (Leptocorisa oratorius Fabricius) and the characters of its volatile compounds. Ann. Res. Rev. Biol. 2020, 35, 34–45. [Google Scholar] [CrossRef]
- Marrison, J.; Räty, L.; Marriott, P.; O’Toole, P. Ptychography—A label free, high-contrast imaging technique for live cells using quantitative phase information. Sci. Rep. 2013, 3, 2369. [Google Scholar] [CrossRef]
- Kasprowicz, R.; Suman, R.; O’Toole, P. Characterising live cell behaviour: Traditional label-free and quantitative phase imaging approaches. Int. J. Biochem. Cell Biol. 2017, 84, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Bellair, C.J.; Harris, P.J.; Allman, B.E.; Roberts, A.; Nugent, K.A.; Delbridge, L.M. Quantitative phase microscopy: A new tool for investigating the structure and function of unstained live cells. Clin. Exp. Pharmacol. Physiol. 2004, 12, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Barer, R. Interference microscopy and mass determination. Nature 1952, 169, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Zangle, T.A.; Teitell, M.A. Live-cell mass profiling: An emerging approach in quantitative biophysics. Nat. Methods 2014, 12, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappaz, B.; Breton, B.; Shaffer, E.; Turcatti, G. Digital Holographic Microscopy: A Quantitative Label-Free Microscopy Technique for Phenotypic Screening. Combinat. Chem. High Throughput Screen. 2014, 17, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Speijer, D. Being right on Q: Shaping eukaryotic evolution. Biochem. J. 2016, 473, 4103–4127. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Trumpower, B.L. The protonmotive Q cycle: Energy transduction by coupling of proton translocation to electron transfer by the cyto-chrome bc1 complex. J. Biol. Chem. 1990, 265, 11409–11412. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, K.; Ema, M.; Suzuki, M.; Yokoyama, C.; Hisatomi, H. Oxidate unsaturated fatty acids induce apoptotic cell death in cultured cells. Mol. Med. Rep. 2019, 19, 2767–2773. [Google Scholar]
- Liu, S.S.; Huang, J.P. Co-existence of “reactive oxygen species” with Q-cycle and proton cycle in respiratory chain of mitochondria. In Proceedings of the International Symposium on Natural Antioxidants: Molecular Mechanisms and Health Effects; Parker, L., Traber, M.G., Xin, W.J., Eds.; AOCS: Champaign, IL, USA, 1996; pp. 511–529. [Google Scholar]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- The American Peony Society: Pink Hawaiian Coral. Available online: https://americanpeonysociety.org/cultivars/peony-registry/pink-hawaiian-coral/ (accessed on 18 January 2021).
- Micheletti, G.; Calonghi, N.; Farruggia, G.; Strocchi, E.; Palmacci, V.; Telese, D.; Bordoni, S.; Frisco, G.; Boga, C. Synthesis of novel structural hybrids between aza-heterocycles and azelaic acid moiety with a specific activity on osteosarcoma cells. Molecules 2020, 25, 404. [Google Scholar] [CrossRef] [Green Version]
- Bergamini, C.; Moruzzi, N.; Sblendido, A.; Lenaz, G.; Fato, R. A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 2012, 7, e33712. [Google Scholar] [CrossRef] [Green Version]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.-Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]






| Compound | r.t. (min.) | SCO Area (%) | SCH Area (%) |
|---|---|---|---|
| Phenol | 17.8 | 0.05 | 0.2 |
| Benzoic acid | 21.8 | 18.4 | 28.2 |
| Palmitic acid | 25.4 | 15.3 | 16.8 |
| Heptadecanoic acid | 26.3 | 0.5 | 0.6 |
| Palmitoleic acid | 26.6 | 0.6 | 0.8 |
| Stearic acid | 27.3 | 0.9 | 1.2 |
| Oleic acid | 27.7 | 5.9 | 8.3 |
| Linoleic acid | 28.3 | 39.6 | 32.6 |
| Methyl linolenate | 29.3 | 5.8 | 3.8 |
| Aplysteryl acetate a | 34.8 | 9.7 | 5.9 |
| Vitamin E | 52.4 | 3.4 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calonghi, N.; Farruggia, G.; Boga, C.; Micheletti, G.; Fini, E.; Romani, L.; Telese, D.; Faraci, E.; Bergamini, C.; Cerini, S.; et al. Root Extracts of Two Cultivars of Paeonia Species: Lipid Composition and Biological Effects on Different Cell Lines: Preliminary Results. Molecules 2021, 26, 655. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030655
Calonghi N, Farruggia G, Boga C, Micheletti G, Fini E, Romani L, Telese D, Faraci E, Bergamini C, Cerini S, et al. Root Extracts of Two Cultivars of Paeonia Species: Lipid Composition and Biological Effects on Different Cell Lines: Preliminary Results. Molecules. 2021; 26(3):655. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030655
Chicago/Turabian StyleCalonghi, Natalia, Giovanna Farruggia, Carla Boga, Gabriele Micheletti, Elena Fini, Lucia Romani, Dario Telese, Erika Faraci, Christian Bergamini, Stefano Cerini, and et al. 2021. "Root Extracts of Two Cultivars of Paeonia Species: Lipid Composition and Biological Effects on Different Cell Lines: Preliminary Results" Molecules 26, no. 3: 655. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030655