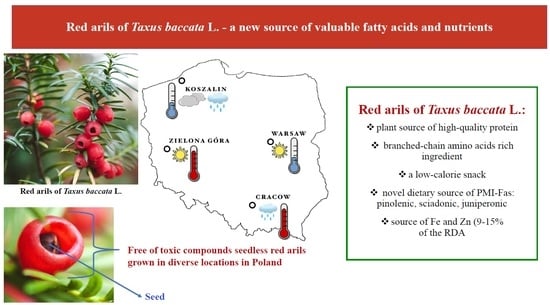
Red Arils of Taxus baccata L.—A New Source of Valuable Fatty Acids and Nutrients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Taxus Compounds
2.2. Proximate Composition
2.3. Amino Acid Profile
2.4. Fatty Acid Composition
2.5. Elemental Characteristic
3. Material and Methods
3.1. Sample Collection
3.2. Analysis of Taxus Compounds
3.3. Analysis of Proximate Composition
3.4. Analysis of Sugars
3.5. Analysis of Amino Acid Profile
3.6. Analysis of Fatty Acid Composition
3.7. Elemental Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hua, Z.; Wang, Z.Y.; Yang, X.; Zhao, H.T.; Zhang, Y.C.; Dong, A.J.; Jing, J.; Wang, J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014, 147, 189–194. [Google Scholar]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Barizza, E.; Guzzo, F.; Fanton, P.; Lucchini, G.; Sacchi, A.G.; Lo Schiavo, F.; Nascimbene, J. Nutritional profile and productivity of bilberry (Vaccinium myrtillus L.) in different habitats of a protected area of the Eastern Italian Alps. J. Food Sci. 2013, 78, C673. [Google Scholar]
- Jaakkola, M.; Korpelainen, V.; Hoppulab, K.; Virtanena, V. Chemical composition of ripe fruits of Rubus chamaemorus L. grown in different habitats. J. Sci. Food Agric. 2012, 92, 1324–1330. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Zheng, Z.Y.; Xu, X.; Hu, Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Guo, M.; Shi, T.; Duan, Y.; Zhu, J.; Li, J.; Cao, Y. Investigation of amino acids in wolfberry fruit (Lycium barbarum) by solid-phase extraction and liquid chromatography with precolumn derivatization. J. Food Compos. Anal. 2015, 42, 84–90. [Google Scholar] [CrossRef]
- He, J.Y.; Zhang, Y.H.; Ma, N.; Zhang, X.L.; Liu, M.H.; Fu, W.M. Comparative analysis multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods. 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Mohamed, A.A.; Ali, S.I.; Alghamdi, N.M.; Abdel-Rahman, A.M.; Al-Sobeai, S. Chemical ingredients and antioxidant activities of underutilized wild fruits. Heliyon 2019, 5, e01874. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.A.; Polwart, A. Taxus baccata L. J. Ecol. 2003, 91, 489–524. [Google Scholar] [CrossRef]
- Milutinović, M.G.; Stanković, M.S.; Cvetković, D.M.; Topuzović, M.D.; Mihailović, V.B.; Marković, S.D. Antioxidant and anticancer properties of leaves and seed cones from European yew (Taxus baccata L.). Arch. Biol. Sci. Belgrade 2015, 67, 525–534. [Google Scholar] [CrossRef]
- Nawrocka-Grześkowiak, U.; Skuza, L.; Szućko, I. Genetic variability of yew in the selected Polish nature reserves. Sylwan 2015, 159, 37–44. [Google Scholar]
- Wilson, C.R.; Sauer, J.M.; Hooser, S.B. Taxines: A review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon 2001, 39, 179–185. [Google Scholar] [CrossRef]
- Glowniak, K.; Mroczek, T.; Zobel, A.M. Seasonal changes in concentrations of four toxoids in Taxus baccata L. during autumn-spring period. Phytomedicine 1999, 6, 135–140. [Google Scholar] [CrossRef]
- Grobosh, T.; Schwarze, B.; Felgenhauer, N.; Riesselmann, B.; Roscher, S.; Binscheck, T. Eight cases of fatal and non-fatal poisoning with Taxus baccata. Forensic Sci. Int. 2013, 227, 118–126. [Google Scholar] [CrossRef]
- Merill, C.; Miller, M.C., III; Johnson, K.R.; Willingham, M.C.; Fan, W. Apoptotic cell death induced by baccatin III, a precursor of paclitaxel, may occur without G2/M arrest. Cancer Chemoter. Pharmacol. 1999, 44, 444–452. [Google Scholar]
- Kucukboyaci, N.; Sener, B. Biological activities of lignans from Taxus baccata L. growing in Turkey. J. Med. Plant Res. 2019, 4, 1136–1140. [Google Scholar]
- Siegle, L.; Pietsch, J. Taxus ingredients in the red arils of Taxus baccata L. determined by HPLC-MS/MS. Phytochem. Anal. 2018, 29, 446–451. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Słupski, J.; Gębczyński, P.; Pogoń, K.; Skoczylas, Ł. Fiber content in aril yew (Taxus baccata L.) originates from different area of Poland. In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 8–10 November 2017; pp. 287–288. [Google Scholar]
- Takagi, T.; Itabashi, Y. cis-5-Olefinic unusual fatty acids in seed lipids of gymnospermae and their dystibution in triacyloglycerols. Lipids 1982, 17, 716–723. [Google Scholar] [CrossRef]
- Wolff, R.; Deluc, L.G.; Marpeau, A.M. Conifer seeds: Oil content and fatty acid composition. J. Am. Oil Chem. Soc. 1996, 73, 765–771. [Google Scholar] [CrossRef]
- Wolff, R.L.; Pédrono, F.; Marpeau, A.M.; Christie, W.W.; Gunstone, F.D. The seed fatty acid composition and the distribution of Δ5-olefinic acids in the triacylglycerols of some taxaceae (Taxus and Torreya). J. Am. Oil Chem. Soc. 1998, 75, 1637–1641. [Google Scholar] [CrossRef]
- Wolff, R.L.; Christie, W.W. Structures, practical sources (gymnosperm seeds), gas-liquid chromatographic data (equivalent chain lengths), and mass spectrometric characteristics of all-cis Δ5-olefinic acids. Eur. J. Lipid Sci. Technol. 2002, 234–244. [Google Scholar] [CrossRef]
- Morishige, J.; Amano, N.; Hirano, K.; Nishio, H.; Tanaka, T.; Satouchi, K. Inhibitory effect of juniperonic acid (Δ-5c, 11c, 14c, 17c - 20:4, Ω-3) on bombesin-induced proliferation of swiss 3T3 cells. Biol. Pham. Bull. 2008, 31, 1786–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.J.; Huang, W.C.; Lin, S.W.; Chen, S.N.; Shen, H.J.; Chang, H.; Chuang, L.T. Juniperonic acid incorporation into the phospholipids of murine macrophage cells modulates pro-inflammatory mediator production. Inflammation 2018, 41, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Hsu, C.P.; Li, C.W.; Lu, J.H.; Chuang, L.T. Pinolenic acid inhibits human breast cancer MDA-MB-231 cell metastasis in vitro. Food Chem. 2011, 126, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Tili, N.; Tir, M.; Benlajnef, H.; Khemiri, S.; Mejri, H.; Rejeb, S.; Khaldi, A. Variation in protein and oil content and fatty acid composition of Rhus tripartitum fruits collected at different maturity stages in different locations. Ind. Crops Prod. 2014, 59, 197–201. [Google Scholar]
- Ma, Y.; Tian, J.; Wang, X.; Huang, C.; Tian, M.; Wei, A. Fatty acid profiling and chemometric analyses for Zantoxylum pericarps from different geographic origin and genotype. Foods 2020, 9, 1676. [Google Scholar] [CrossRef]
- Madawala, S.R.P.; Brunius, C.; Adholeya, A.; Tripathi, S.B.; Hanhineva, K.; Hajazimi, E.; Shi, L.; Dimberg, L.; Landberg, R. Impact of location on composition of selected phytochemicals in wild sea buckthorn (Hippophae rhamnoides). J. Food Compos. Anal. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Salvador, Ȃ.C.; Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Silvestre, A.J.D.; Rocha, S.M. Bioactive phytochemicals from wild Arbutus unedo L. berries from different locations in Portugal: Quantification of lipophilic components. Int. J. Mol. Sci. 2015, 16, 14194–14209. [Google Scholar] [CrossRef] [Green Version]
- Iszkuło, G.; Kosiński, P.; Hajnos, M. Sex influences the taxanes content in Taxus baccata. Acta Physiol. Plant. 2013, 35, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Veselá, D.; Šaman, D.; Valterová, I.; Vanĕk, T. Seasonal variations in the content of taxanes in the bark of Taxus baccata L. Phytochem. Anal. 1999, 10, 319–321. [Google Scholar]
- Sharma, H.; Garg, M. A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. J. Integr. Med. 2015, 13, 80–90. [Google Scholar] [CrossRef]
- Gai, Q.Y.; Jiao, J.; Wang, X.; Liu, J.; Fu, Y.J.; Lu, Y.; Wang, Z.Y.; Xu, X.J. Simultaneous determination of taxoids and flavonoids in twigs and leaves of three Taxus species by UHPLC-MS/MS. J. Pharmaceut. Biomed. 2020, 189, 113456. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Rodriguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Rutkowska, J.; Adamska, A.; Pielat, M.; Białek, M. Comparison of composition and properties of Rosa rugosa fruits preserved using conventional and freeze-drying methods. Zywn-Nauk. Technol. Ja. 2012, 4, 32–43. [Google Scholar] [CrossRef]
- Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food Anal. Methods 2018, 11, 1180–1188. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Chemical characterization and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kitaura, Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, A.C.; Sánchez-Mata, M.C.; Pérez-Rodríguez, M.L.; Cámara, M.; López-Colón, J.L.; Bach, F.; Bellettini, M.; Haminiuk, I.C.W. Qualitative and nutritional comparison of goji berry fruits produced in organic and conventional systems. Sci. Hortic. 2019, 257, 108660. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Ben-Hassine, K.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammamia, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Patil, M.M.; Muhammed, A.M.; Anu-Appaiah, K.A. Lipids and fatty acid profiling of major Indian Garcinia fruit: A comparative study and its nutritional impact. J. Am. Oil Chem. Soc. 2016, 93, 823–836. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, J.; Qin, M.; Wang, J.; Jiang, B.; Yang, H.; Zhang, Y. Fractionated antioxidant and anti-inflammatory kernel oil from Torreya fargesii. Molecules 2019, 24, 3402. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Salcedo, E.M.; Sendra, E.; Carbonell-Barrachina, A.A.; Martinez, J.J.; Hernandez, F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016, 190, 566–571. [Google Scholar] [CrossRef]
- Nowak-Dyjeta, K.; Giertych, M.J.; Thomas, P.; Iszkuło, G. Males and females of Juniperus communis L. and Taxus baccata L. show different seasonal patterns of nitrogen and carbon content in needles. Acta Physiol. Plant. 2017, 191. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Hardisson, A.; Rubio, C.; Baez, A.; Martin, M.; Alvarez, R.; Diaz, E. Mineral composition of banana (Musa acuminate) from the island of Tenerife. Food Chem. 2001, 73, 153–161. [Google Scholar] [CrossRef]
- Jarosz, M. (Ed.) Nutritional Standards for the Polish Population; Institute of Food and Nutrition: Warsaw, Poland, 2017; pp. 238–256. [Google Scholar]
- Carrigan, A.; Klinger, A.; Choquette, S.S.; Luzuriaga-McPherson, A.; Bell, E.K.; Darnell, B.; Gutiéerrez, O.M. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J. Ren. Nutr. 2014, 24, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heghedüs-Mindru, R.C.; Heghedüs-Mindru, G.; Negrea, P.; Sumalan, R.; Negrea, A.; Stef, D. The monitoring of mineral elements content in fruit purchased in supermarkets and food markets in Timisoara, Romania. Ann. Agric. Environ. Med. 2014, 21, 98–105. [Google Scholar]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, L. Nothing boring about Boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta 2012, 730, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ekholm, P.; Reinivuo, H.; Mattila, P.; Pakkala, H.; Koponen, J.; Happonen, A.; Hellstroöm, J.; Ovaskainen, M.L. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Compos. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- EFSA-European Food Safety Authority, Statement on the evaluation of a new study related to the bioavailability of aluminium in food. EFSA J. 2011, 9, 21–57.
- EFSA-European Food Safety Authority, Cadmium in food Scientific opinion of the Panel on Contaminants in the Food Chain Scientific Opinion. EFSA J. 2009, 980, 1–139.
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Comm. 2006, 364, 5–24.
- Rose, M.; Baxter, M.; Brereton, N.; Baskaran, C. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit. Contam. 2010, 10, 1380–1404. [Google Scholar] [CrossRef] [Green Version]
- Seneta, W. Conifer trees and shrubs, part II, 2nd ed.; PWN: Warsaw, Poland, 1987; pp. 455–456. [Google Scholar]
- Krüssmann, G. Handbook of Conifers, 2nd Revised ed. With the Participation of Hans-Dieter Warda; Paul Parey Publishing House: Berlin/Hamburg, Germany, 1983; pp. 318–319. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC International: Arlington, VA, USA, 1995; Volume 2. [Google Scholar]
- Dhillon, M.K.; Kumar, S.; Gujar, G.T. A common HPLC-PDA method for amino acid analysis in insects and plants. Indian J. Exp. Biol. 2014, 52, 73–79. [Google Scholar]
- Pasławski, P.; Migaszewski, Z.M. The quality of element determinations implant materials by instrumental methods. Pol. J. Environ. Stud. 2006, 15, 154–164. [Google Scholar]


| Compound | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| 10-Deacetylbaccatin III | 19.80 ± 0.64 c | 3.90 ± 0.13 a | 7.40 ± 0.29 b | 4.10 ± 0.18 a |
| Baccatin III | 6.30 ± 0.13 c | 2.00 ± 0.07 a | 2.30 ± 0.03 a,b | 2.40 ± 0.06 b |
| Cephalomannine | 0.05 ± 0.00 b | 0.18 ± 0.01 c | 0.12 ± 0.01 a | 0.12 ± 0.00 a |
| Taxinine M | 0.13 ± 0.01 d | 0.05 ± 0.00 c | 0.03 ± 0.00 b | 0.02 ± 0.00 a |
| Taxol A | 0.02 ± 0.00 b | 0.10 ± 0.00 a | 0.05 ± 0.00 c | 0.05 ± 0.00 c |
| Growth Site Characteristics | ||||
|---|---|---|---|---|
| Exposition | West | Central | North | South |
| Location of natural habitats | Zielona Góra | Warsaw | Koszalin | Cracow |
| Latitude/longitude | 51°56′N 15°30′E | 52°13′N 21°01′E | 54°11′N 16°11′E | 50°05′N 19°58′E |
| Altitude above sea level | 140 m | 90 m | 40 m | 240 m |
| Soil Parameters | ||||
| Type of soil | brown earths soil | podsolic soil | acidic brown soil | alluvial soil |
| Soil pH | >5.6 | 5.26–5.5 | <4.5 | 5–5.25 |
| Mineral nitrogen (mg/kg) | 75.1–113.2 | <10 | 20.1–35 | 20.1–35 |
| Phosphorus availability (mg P2O5/100 g) | 15–20 | 10–15 | >25 | 10–15 |
| Potassium availability (mg K2O/100 g) | 15–20 | <15 | <15 | 15–20 |
| Magnesium availability (mg Mg/100 g) | 6–7 | <5 | 5–6 | >10 |
| Weather Conditions | ||||
| Average annual temperature (°C) | 8.8 | 7.7 | 7.9 | 8.2 |
| Average temperature (°C) from flowering to sampling * | 13 | 12.1 | 11.7 | 12.6 |
| Total annual precipitation (mm) | 572 | 501 | 683 | 678 |
| Average precipitation (mm) from flowering to sampling * | 416 | 385 | 490 | 536 |
| Cloudiness (okta) | 5.6 | 5.7 | 5.9 | 5.3 |
| Exposure to solar radiation (h) | 1700–1800 | 1700–1800 | 1700–1800 | 1600–1700 |
| Intensity of light (W/m2) | 120–140 | 120–140 | 110–130 | 110–130 |
| Component | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| Macronutrients (g/100 g of fresh weight) | ||||
| Proteins | 1.79 ± 0.02 a | 3.80 ± 0.20 c | 3.03 ± 0.10 b | 1.95 ± 0.17 a |
| Lipids | 1.39 ± 0.12 b | 0.79 ± 0.04 a | 3.55 ± 1.24 c | 3.54 ± 1.11 c |
| Carbohydrates | 18.49 ± 0.13 a | 19.06 ± 0.16 a | 19.30 ± 0.22 a | 18.43 ± 0.10 a |
| Glucose | 2.73 ± 0.09 b | 3.03 ± 0.12 c | 2.23 ± 0.12 a | 2.22 ± 0.02 a |
| Fructose | 5.78 ± 0.19 a,b | 6.43 ± 0.27 b | 5.55 ± 0.33 b | 5.36 ± 0.04 a |
| Sucrose | 2.28 ± 0.07 c | 1.65 ± 0.05 b | 0.92 ± 0.03 a | 2.65 ± 0.09 d |
| Moisture (%) * | 77.90 ± 0.09 c | 75.89 ± 0.0.07 b | 73.63 ± 0.08 a | 75.71 ± 0.11 b |
| Dry matter (%) * | 22.10 ± 0.06 a | 24.11 ± 0.17 b | 26.37 ± 0.05 c | 24.29 ± 0.03 b |
| Ash (%) * | 0.43 ± 0.01 b | 0.46 ± 0.01 b,c | 0.49 ± 0.01 c | 0.37 ± 0.02 a |
| Energy value (kcal) | 93.63 ± 0.15 a | 98.55 ± 0.21b | 121.27 ± 0.24 d | 113.38 ± 0.12 c |
| Amino Acids | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| Essential amino acids | ||||
| Histidine | 80.0 ± 0.0 c | 90.0 ± 0.0 d | 43.3 ± 3.3 a | 50.0 ± 0.0 b |
| Threonine | 80.0 ± 0.0 b | 110.0 ± 0.0 c | 80.0 ± 0.0 b | 73.3 ± 3.3 a |
| Tyrosine | 40.0 ± 0.0 b | 53.3 ± 3.3 c | 30.0 ± 0.0 a | 30.0 ± 0.0 a |
| Valine | 130.0 ± 0.0 c | 166.7 ± 3.3 d | 106.7 ± 3.3 b | 90.0 ± 0.0 a |
| Methionine and cysteine | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| Lysine | 110.0 ± 0.0 a | 170.0 ± 0.0 c | 143.3 ± 3.3 b | 110.0 ± 0.0 a |
| Isoleucine | 100.0 ± 0.0 c | 120.0 ± 0.0 d | 73.3 ± 3.3 b | 63.3 ± 3.3 a |
| Leucine | 183.3 ± 3.3 c | 220.0 ± 0.0 d | 133.3 ± 3.3 b | 123.3 ± 3.3 a |
| Phenylalanine | 173.3 ± 3.3 c | 176.7 ± 3.3 c | 70.0 ± 0.0 a | 80.00 ± 0.0 b |
| Total | 996.6 ± 0.7 c | 1206.7 ± 1.0 d | 779.9 ± 1.7 b | 719.9 ± 1.0 a |
| Non-essential amino acids | ||||
| Aspartic acid | 90.0 ± 0.0 a | 143.0 ± 3.3 c | 147.0 ± 3.3 c | 113.0 ± 3.3 b |
| Serine | 170.0 ± 0.0 b | 236.7 ± 3.3 c | 153.3 ± 3.3 a | 153.3 ± 3.3 a |
| Glutamic acid | 200.0 ± 0.0 a | 510.0 ± 5.7 d | 316.7 ± 6.7 c | 283.3 ± 3.3 b |
| Glycine | 126.7 ± 3.3 c | 146.7 ± 3.3 d | 80.0 ± 0.0 a | 90.0 ± 0.0 b |
| Arginine | 70.0 ± 0.0 a | 100.0 ± 0.0 c | 90.0 ± 0.0 b | 90.0 ± 0.0 b |
| Alanine | 110.0 ± 0.0 a | 226.7 ± 3.3 c | 236.7 ± 6.7 c | 203.3 ± 3.3 b |
| Proline | 150.0 ± 0.0 a | 220.0 ± 0.0 b | 210.0 ± 5.8 b | 153.3 ± 3.3 a |
| Total | 916.7 ± 0.5 a | 1583.1 ± 2.7 d | 1233.7 ± 3.7 c | 1086.2 ± 2.4 b |
| Fatty Acid | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| SFAs | 33.33 ± 0.14 a | 38.39 ± 0.15 b | 33.12 ± 0.31 a | 38.51 ± 0.04 b |
| 10:0 | 0.05 ± 0.00 a | 0.12 ± 0.01 b | 0.07 ± 0.00 a | 0.13 ± 0.00 c |
| 12:0 | 0.50 ± 0.01 b | 1.13 ± 0.01 c | 0.30 ± 0.02 a | 0.51 ± 0.02 b |
| 14:0 | 9.84 ± 0.06 b | 10.76 ± 0.05 d | 6.76 ± 0.04 a | 10.39 ± 0.07 c |
| 16:0 | 20.43 ± 0.10 a | 22.66 ± 0.29 b | 22.37 ± 0.31 b | 24.37 ± 0.10 c |
| 17:0 | 0.14 ± 0.01 c | 0.12 ± 0.01 b | 0.13 ± 0.00 b,c | 0.08 ± 0.00 a |
| 18:0 | 1.88 ± 0.02 a | 2.62 ± 0.07 c | 3.37 ± 0.03 d | 2.19 ± 0.03 b |
| 22:0 | 0.16 ± 0.00 a | 0.18 ± 0.00 d | 0.11 ± 0.01 c | 0.24 ± 0.01 b |
| 24:0 | 0.32 ± 0.02 b | 0.81 ± 0.03 d | 0.00 ± 0.00 a | 0.59 ± 0.01 c |
| MUFAs | 10.71 ± 0.07 c | 8.71 ± 0.08 b | 13.04 ± 0.04 d | 7.69 ± 0.06 a |
| 14:1 | 0.13 ± 0.00 a | 0.16 ± 0.02 c | 0.15 ± 0.00 b | 0.26 ± 0.01 d |
| 16:1Δ7 | 0.12 ± 0.01 a | 0.27 ± 0.00 b | 0.12 ± 0.00 a | 0.35 ± 0.01 c |
| 16:1Δ9 | 0.20 ± 0.01 b | 0.29 ± 0.00 d | 0.26 ± 0.00 c | 0.16 ± 0.00 a |
| 17:1 | 0.11 ± 0.01 a | 0.85 ± 0.03 c | 0.14 ± 0.01 a | 0.28 ± 0.02 b |
| 18:1Δ9 | 9.52 ± 0.06 b | 6.65 ± 0.08 a | 12.35 ± 0.06 c | 6.59 ± 0.10 a |
| 18:1Δ11 | 0.39 ± 0.02 c | 0.37 ± 0.03 c | 0.00 ± 0.00 a | 0.07 ± 0.00 b |
| 20:1Δ9 | 0.24 ± 0.01 d | 0.11 ± 0.00 c | 0.04 ± 0.00 b | 0.00 ± 0.00 a |
| n-3-PUFAs | 19.19 ± 0.17 a | 25.85 ± 0.11 c | 24.18 ± 0.20 b | 28.19 ± 0.17 d |
| 18:3Δ9,12,15 | 18.53 ± 0.18 a | 25.18 ± 0.14 c | 23.43 ± 0.23 b | 26.50 ± 0.19 d |
| 20:3Δ11,14,17 | 0.67 ± 0.03 a | 0.67 ± 0.01 a | 0.76 ± 0.02 b | 1.69 ± 0.02 c |
| n-6-PUFAs | 33.81 ± 0.16 c | 24.24 ± 0.20 b | 25.19 ± 0.16 b | 21.00 ± 0.13 a |
| 18:2Δ9,12 | 30.92 ± 0.12 c | 20.99 ± 0.22 b | 21.33 ± 0.11 b | 19.40 ± 0.11 a |
| 18:3Δ6,9,12 | 0.63 ± 0.03 b | 0.71 ± 0.01 c | 0.80 ± 0.02 d | 0.50 ± 0.02 a |
| 20:2Δ11,14 | 0.16 ± 0.01 a | 0.14 ± 0.00 a | 0.16 ± 0.00 a | 0.62 ± 0.02 b |
| 22:2Δ13,16 | 1.62 ± 0.03 b | 1.96 ± 0.01 c | 2.33 ± 0.04 d | 0.07 ± 0.00 a |
| 20:4Δ8,11,14,17 | 0.48 ± 0.02 b | 0.44 ± 0.01 a,b | 0.57 ± 0.02 c | 0.41 ± 0.01 a |
| PMI-FAs | 1.41 ± 0.01 b | 1.32 ± 0.01 b | 1.08 ± 0.00 a | 0.98 ± 0.01 a |
| 18:3Δ5, 9,12 | 0.09 ± 0.01 a | 0.14 ± 0.00 b | 0.08 ± 0.00 a | 0.23 ± 0.01 c |
| 20:3Δ5,11,14 | 1.25 ± 0.03 d | 0.94 ± 0.02 c | 0.82 ± 0.02 b | 0.62 ± 0.02 a |
| 20:4Δ5,11,14,17 | 0.06 ± 0.00 b | 0.24 ± 0.00 b | 0.17 ± 0.00 a | 0.12 ± 0.00 c |
| PUFAs | 54.41 ± 0.23 c | 51.40 ± 0.12 b | 50.45 ± 0.16 a | 50.16 ± 0.08 a |
| NI | 1.55 ± 0.32 a | 1.50 ± 0.21 a | 3.38 ± 0.45 b | 3.63 ± 0.15 b |
| Fatty Acid | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| n-3-PUFAs | 267.79 ± 1.73 b | 204.19 ± 0.85 a | 858.51 ± 6.77 c | 997.81 ± 5.12 d |
| 18:3Δ9,12,15 | 257.52 ± 2.05 b | 198.90 ± 0.91 a | 831.65 ± 6.62 c | 937.98 ± 5.43 d |
| 20:3Δ11,14,17 | 9.27 ± 0.33 b | 5.29 ± 0.06 a | 26.86 ± 0.54 c | 59.83 ± 0.60 d |
| n-6-PUFAs | 469.96 ± 1.35 b | 191.47 ± 1.46 a | 894.36 ± 3.86 d | 743.40 ± 3.74 c |
| 18:2Δ9,12 | 429.74 ± 1.31 b | 165.82 ± 1.44 a | 757.33 ± 3.28 d | 686.64 ± 3.08 c |
| 18:3Δ6,9,12 | 8.76 ± 0.36 b | 5.61 ± 0.04 a | 28.40 ± 0.60 d | 17.70 ± 0.44 c |
| 20:2Δ11,14 | 2.27 ± 0.08 b | 1.08 ± 0.02 a | 5.80 ± 0.10 c | 21.83 ± 0.59 d |
| 22:2Δ13,16 | 22.56 ± 0.36 c | 15.51 ± 0.04 b | 82.72 ± 1.10 d | 2.60 ± 0.10 a |
| n-6/n-3 | 1:1.7 d | 1:1 b | 1:1 b | 1:0.8 a |
| PMI-Fas * | 19.55 ± 0.46 b | 10.43 ± 0.10 a | 38.22 ± 0.59 d | 34.57 ± 0.42 c |
| 18:3Δ5,9,12 | 1.25 ± 0.07 a | 1.13 ± 0.02 a | 2.96 ± 0.10 b | 8.14 ± 0.33 c |
| 20:3Δ5,11,14 | 17.42 ± 0.36 b | 7.43 ± 0.13 a | 29.23 ± 0.42 d | 22.07 ± 0.42 c |
| 20:4Δ5,11,14,17 | 0.88 ± 0.04 a | 1.87 ± 0.06 b | 6.04 ± 0.17 d | 4.37 ± 0.10 c |
| Total PUFAs | 756.30 ± 3.26 b | 406.09 ± 0.94 a | 1791.09 ± 5.78 c | 1775.78 ± 2.84 c |
| Element | Fruit Collection Site | |||
|---|---|---|---|---|
| Zielona Góra | Warsaw | Koszalin | Cracow | |
| Macroelements (mg/100 g of fresh weight) | ||||
| K | 772.29 ± 26.27 a | 878.38 ± 5.14 b | 838.18 ± 41.19 a | 789.62 ± 50.86 a |
| P | 95.96 ± 1.76 a | 109.35 ± 6.14 a | 101.03 ± 6.24 a | 97.89 ± 7.08 a |
| S | 29.13 ± 1.38 a,b | 30.99 ± 2.31 b | 27.78 ± 1.07 a,b | 24.97 ± 0.52 a |
| Ca | 20.75 ± 0.49 a | 21.05 ± 0.53 a | 23.69 ± 0.09 b | 19.88 ± 0.33 a |
| Mg | 19.53 ± 0.28 a | 25.71 ± 0.58 c | 22.88 ± 0.47 b | 24.63 ± 0.34 c |
| Na | 1.12 ± 0.20 a | 4.90 ± 1.26 c | 2.81 ± 0.20 b | 0.86 ± 0.39 a |
| Microelements (μg/100 g of fresh weight) | ||||
| Zn | 1506.54 ± 16.98 b | 947.72 ± 32.46 a | 1146.59 ± 89.23 a | 1080.24 ± 56.71 a |
| Fe | 1111.39 ± 28.67 a | 1447.57 ± 80.42 b | 2537.13 ± 142.95 c | 976.19 ± 68.97 a |
| B | 619.40 ± 2.61 b | 1152.07 ± 11.10 c | 463.87 ± 63.22 a | 652.87 ± 50.63 b |
| Cu | 240.53 ± 1.24 b | 251.23 ± 24.40 a | 206.10 ± 19.21 a | 201.84 ± 16.15 a |
| Mn | 103.87 ± 1.93 b | 76.45 ± 2.62 a | 521.09 ± 5.35 c | 721.55 ± 13.56 d |
| Cr | 15.42 ± 0.64 b,c | 16.36 ± 1.60 c | 13.57 ± 1.16 b,c | 11.18 ± 0.53 a |
| Mo | 11.06 ± 0.43 a | 17.68 ± 0.28 b | 11.43 ± 0.03 a | 17.85 ± 0.48 b |
| Co | 7.34 ± 0.21 b | 0.13 ± 0.03 a | 7.53 ± 0.09 b | 6.03 ± 0.17 b |
| Metals (μg/100 g of fresh weight) | ||||
| Al | 466.48 ± 24.80 b | 616.56 ± 17.23 c | 1855.06 ± 148.40 d | 306.63 ± 7.16 a |
| Ni | 129.52 ± 1.68 a | 98.93 ± 8.02 a | 409.77 ± 4.23 c | 217.53 ± 4.87 b |
| Bi | 62.65 ± 0.29 b,c | 54.15 ± 14.44 b | 46.02 ± 21.56 b | 22.17 ± 17.43 a |
| Ba | 37.21 ± 1.23 b | 34.18 ± 1.79 b | 77.64 ± 12.40 c | 24.63 ± 0.41 a |
| In | 33.40 ± 2.48 b | 23.92 ± 3.18 a | 19.24 ± 2.84 a | 22.52 ± 2.79 a |
| Ti | 32.55 ± 3.35 b | 47.67 ± 1.03 c | 71.12 ± 3.81 d | 17.03 ± 2.26 a |
| Li | 13.40 ± 0.53 c | 22.02 ± 0.46 d | 10.42 ± 0.58 b | 3.16 ± 0.29 a |
| Ag | 14.32 ± 1.50 a | 12.85 ± 0.93 a | 11.80 ± 0.65 a | 12.17 ± 0.54 a |
| Cd | 7.41 ± 2.46 b | 9.21 ± 1.09 b | 4.78 ± 0.52 a | 24.66 ± 3.48 c |
| Ga | 7.29 ± 0.34 b | 11.62 ± 0.90 c | 16.21 ± 1.14 d | 2.40 ± 0.61 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabaszewska, M.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Antoniewska, A.; Smoleń, S.; Łukasiewicz, M.; Baranowski, D.; Duda, I.; Pietsch, J. Red Arils of Taxus baccata L.—A New Source of Valuable Fatty Acids and Nutrients. Molecules 2021, 26, 723. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030723
Tabaszewska M, Rutkowska J, Skoczylas Ł, Słupski J, Antoniewska A, Smoleń S, Łukasiewicz M, Baranowski D, Duda I, Pietsch J. Red Arils of Taxus baccata L.—A New Source of Valuable Fatty Acids and Nutrients. Molecules. 2021; 26(3):723. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030723
Chicago/Turabian StyleTabaszewska, Małgorzata, Jaroslawa Rutkowska, Łukasz Skoczylas, Jacek Słupski, Agata Antoniewska, Sylwester Smoleń, Marcin Łukasiewicz, Damian Baranowski, Iwona Duda, and Jörg Pietsch. 2021. "Red Arils of Taxus baccata L.—A New Source of Valuable Fatty Acids and Nutrients" Molecules 26, no. 3: 723. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030723