Systematic Phytochemical Screening of Different Organs of Calotropis procera and the Ovicidal Effect of Their Extracts to the Foodstuff Pest Cadra cautella
Abstract
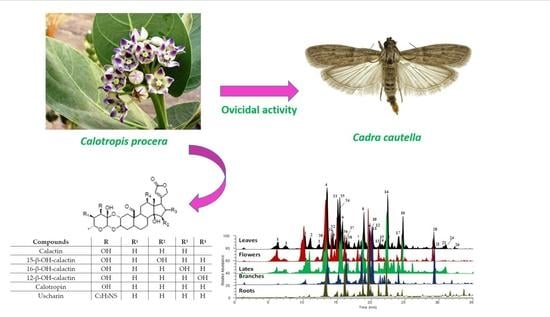
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Extracts
2.2. LC-MS/MS Analysis
2.3. Quantitative Analysis
2.4. C. procera Extracts Ovicidal Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Methods
3.3. General Experimental Procedures
3.4. Qualitative Analysis, LC-ESI-OrbitrapMS
3.5. Quantitative Analysis
3.6. Ovicidal Activity of C. procera Extracts on Cadra Cautella
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef]
- Jiao, Z.; Wu, Y.; Qu, S. Fenpropathrin induces degeneration of dopaminergic neurons via disruption of the mitochondrial quality control system. Cell Death Discov. 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Garry, V.F. Pesticides and children. Toxicol. Appl. Pharmacol. 2004, 198, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Martin-Guay, M.O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wilcock, C.C. A taxonomic revision of Calotropis (Asclepiadaceae). Nord. J. Bot. 1991, 11, 301–308. [Google Scholar] [CrossRef]
- Sweidan, N.I.; Abu Zarga, M.H. Two novel cardenolides from Calotropis procera. J. Asian Nat. Prod. Res. 2015, 17, 900–907. [Google Scholar] [CrossRef]
- Dhalendra, G.; Rathore, P.; Satapathy, T.; Roy, A. Pharmacognostical, phytochemical and pharmacological study of Calotropis procera: A review. Res. J. Pharm. Technol. 2014, 7, 346–351. [Google Scholar]
- Chundattu, S.J.; Agrawal, V.K.; Ganesh, N. Phytochemical investigation of Calotropis procera. Arab. J. Chem. 2016, 9, S230–S234. [Google Scholar] [CrossRef] [Green Version]
- Meena, A.K.; Yadav, A.; Rao, M. Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J. Tradit. Med. 2011, 6, 45–53. [Google Scholar]
- Sharma, P.; Sharma, J.D. Evaluation of in vitro schizontocidal activity of plant parts of Calotropis procera—An ethnobotanical approach. J. Ethnopharmacol. 1999, 68, 83–95. [Google Scholar] [CrossRef]
- Rao, K.V.B.; Kumar, G.; Karthik, L.; Kirthi, A.V.; Jayaseelan, C.; Rahuman, A.A. Phytochemical composition, mosquito larvicidal, ovicidal and repellent activity of Calotropis procera against Culex tritaeniorhynchus and Culex gelidus. Bangladesh J. Pharmacol. 2012, 7, 63–69. [Google Scholar]
- Singh, R.; Mittal, P.; Dhiman, R. Laboratory study on larvicidal properties of leaf extract of Calotropis procera (Family-Asclepiadaceae) against mosquito larvae. J. Commun. Dis. 2005, 37, 109. [Google Scholar] [PubMed]
- Shahi, M.; Hanafi-Bojd, A.; Iranshahi, M.; Vatandoost, H.; Hanafi-Bojd, M. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J. Vector Borne Dis. 2010, 47, 185–188. [Google Scholar]
- Su, T.; Mulla, M.S. Antifeedancy of neem products containing Azadirachtin against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J. Soc. Vector Ecol. 1998, 23, 114–122. [Google Scholar]
- Elimam, A.M.; Elmalik, K.H.; Ali, F.S. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci. 2009, 16, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Elamir, E.E.; Almadiy, A.A.; Nenaah, G.E.; Alabas, A.A.; Alsaqri, H.S. Comparing six mathematical link function models of the antifeedant activity of lesser grain borer exposed to sub-lethal concentrations of some extracts from Calotropis procera. Bioengineered 2019, 10, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Farrar, N.; Golestaneh, S.; Farsi, M.; Sadeghi, S.; Askari, H. Effectiveness of Extract of Calotropis procera on Nutritional Indices of Tribolium confusum Duv. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on the 940, Lisboa, Portugal, 22–27 August 2010; pp. 669–672. [Google Scholar]
- Khaliq, A.; Ullah, M.I.; Afzal, M.; Ali, S.; Sajjad, A.; Ahmad, A.; Khalid, S. Management of Tribolium castaneum using synergism between conventional fumigant and plant essential oils. Int. J. Trop. Insect Sci. 2020, 40, 781–788. [Google Scholar] [CrossRef]
- Zahran, M.; Younes, H.; Al-Tawil, B. Ecology of four community types: Red Sea coastal desert, Saudi Arabia. J. Coast. Res. 1985, 1, 279–288. [Google Scholar]
- Siciliano, T.; Leo, M.D.; Bader, A.; Tommasi, N.D.; Vrieling, K.; Braca, A.; Morelli, I. Pyrrolizidine alkaloids from Anchusa strigosa and their antifeedant activity. Phytochemistry 2005, 66, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [Green Version]
- Begum, N. Calotropis procera and Annona squamosa: Potential Alternatives to Chemical Pesticides. Br. J. Appl. Sci. Technol. 2013, 3, 254–267. [Google Scholar] [CrossRef]
- Nenaah, G. Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J. Microbiol. Biotechnol. 2013, 29, 1255–1262. [Google Scholar] [CrossRef]
- Begum, N.; Sharma, B.; Pandey, R. Evaluation of Insecticidal Efficacy of Calotropis Procera and Annona Squamosa Ethanol Extracts against Musca Domestica. J. Biofertil. Biopestic. 2010, 1, 2–6. [Google Scholar] [CrossRef]
- Roy, S.; Handique, G.; Muraleedharan, N.; Dashora, K.; Roy, S.M.; Mukhopadhyay, A.; Babu, A. Use of plant extracts for tea pest management in India. Appl. Microbiol. Biotechnol. 2016, 100, 4831–4844. [Google Scholar] [CrossRef]
- Patil, S.V.; Patil, C.D.; Salunkhe, R.B.; Salunke, B.K. Larvicidal activities of six plants extracts against two mosquito species, Aedes aegypti and Anopheles stephensi. Trop. Biomed. 2010, 27, 360–365. [Google Scholar]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 17857. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 135, 581–598. [Google Scholar]
- Tahir, M.; Ishaq, T.; Mukhtar, M.; Khan, S. Potential use of Calotropis procera (Milk Weed) to Control Culex quinquefasciatus (Diptera: Culicidae). Pak. J. Zool. 2013, 45, 615–621. [Google Scholar]
- Ranjit, P.M.; Eswara Rao, G.; Krishnapriya, M.; Nagalakshmi, V.; Silpa, P.; Anjali, M. An overview of phytochemical and pharmacological activities of Calotropis procera. FS J. Pharm. Res. 2012, 1, 18–25. [Google Scholar]
- Pandey, A.; Swarnkar, V.; Pandey, T.; Srivastava, P.; Kanojiya, S.; Mishra, D.K.; Tripathi, V. Transcriptome and Metabolite analysis reveal candidate genes of the cardiac glycoside biosynthetic pathway from Calotropis procera. Sci. Rep. 2016, 6, 34464. [Google Scholar] [CrossRef] [Green Version]
- Hanna, A.G.; Shalaby, N.M.; Morsy, N.A.; Simon, A.; Toth, G.; Malik, S.; Duddeck, H. Structure of a calotropagenin-derived artifact from Calotropis procera. Magn. Reson. Chem. 2002, 40, 599–602. [Google Scholar] [CrossRef]
- Kanojiya, S.; Madhusudanan, K.P. Rapid identification of calotropagenin glycosides using high-performance liquid chromatography electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2012, 23, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; JN, S.; CJ, N. Cardenolides in the latex and leaves of seven Asclepias species and Calotropis procera. Phytochemistry 1982, 21, 2343–2348. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Liu, M.; Abdel-Mageed, W.M.; Alwahibi, L.H.; Dai, H.; Ismail, M.A.; Badr, G.; Quinn, R.J.; Liu, X.; Zhang, L.; et al. Cytotoxic cardenolides from the latex of Calotropis procera. Bioorg. Med. Chem. Lett. 2015, 25, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Parihar, G.; Balekar, N. Calotropis procera: A phytochemical and pharmacological review. Thai J. Pharm. Sci. 2016, 40, 1–17. [Google Scholar]
- Crout, D.; Hassall, C.; Jones, T. 405. Cardenolides. Part VI. Uscharidin, calotropin, and calotoxin. J. Chem. Soc. 1964, 2187–2194. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Basha, A. Spectroscopic Data of Steroid Glycosides: Spirostanes, Bufanolides, Cardenolides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; Volume 3. [Google Scholar]
- Hassall, C.; Reyle, K. 18. Cardenolides. Part III. The constitution of calotropagenin. J. Chem. Soc. 1959, 85–89. [Google Scholar] [CrossRef]
- Elgamal, M.H.A.; Hanna, A.G.; Morsy, N.A.; Duddeck, H.; Simon, A.; Gáti, T.; Tóth, G. Complete 1H and 13C signal assignments of 5α-cardenolides isolated from Calotropis procera R. BR. J. Mol. Struct. 1999, 477, 201–208. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Shaala, L.A.; Moreno, L.; Banuls, Y.; Kiss, R.; Youssef, D.T. Proceraside A, a new cardiac glycoside from the root barks of Calotropis procera with in vitro anticancer effects. Nat. Prod. Res. 2014, 28, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Crout, D.; Curtis, R.; Hassall, C. 347. Cardenolides. Part V. The constitution of calactinic acid. J. Chem. Soc. 1963, 1866–1875. [Google Scholar] [CrossRef]
- Van Quaquebeke, E.; Simon, G.; André, A.; Dewelle, J.; El Yazidi, M.; Bruyneel, F.; Tuti, J.; Nacoulma, O.; Guissou, P.; Decaestecker, C.; et al. Identification of a novel cardenolide (2″-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: Structure-activity relationship analyses. J. Med. Chem. 2005, 48, 849–856. [Google Scholar] [CrossRef]
- Shaker, K.H.; Morsy, N.; Zinecker, H.; Imhoff, J.F.; Schneider, B. Secondary metabolites from Calotropis procera (Aiton). Phytochem. Lett. 2010, 3, 212–216. [Google Scholar] [CrossRef]
- Bedini, S.; Farina, P.; Napoli, E.; Flamini, G.; Ascrizzi, R.; Verzera, A.; Conti, B.; Zappalà, L. Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens. Insects 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Hamed, M.M.; Ahmed, W.S.; Abdou, A.M. Antioxidant and cytotoxic flavonols from Calotropis procera. Z. Nat. C 2011, 66, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Matharu, K.; Mehta, P. Ovicidal activity of crude extracts of indigenous plant species against Plutella xylostella (L.) (Lepidoptera: Plutellidae). Environ. Ecol. 2017, 35, 285–289. [Google Scholar]
- Alves, D.S.; Oliveira, D.F.; Carvalho, G.A.; Santos, H.M.; Carvalho, D.A.; Santos, M.A.; Carvalho, H.W. Plant extracts as an alternative to control Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae). Neotrop. Entomol. 2011, 40, 123–128. [Google Scholar] [CrossRef] [Green Version]





| No | Formula | [M − H]+ | MS/MS | Compound and CAS Number | Organs and Latex | Literature |
|---|---|---|---|---|---|---|
| Cardenolides | ||||||
| 1 | C23H32O7 | 421.2209 | 403 [M+H-18]+ 385 [M+H-18-18]+ 367 [M+H-18-18-18]+ 339 [M+H-18-18-28-18]+ | Hydroxycalotropagenin isomer b tentatively identified | Latex, flowers, leaves, branches, roots | [34] |
| 2 | C29H40O10 | 549.2622 | 531 [M+H-18]+ 513 [M+H-18-18]+ 485 [M+H-18-18-28]+ 467 [M+H-18-18-28-18]+ 385 [M+H-146-18]+ 367 [M+H-146-18-18]+ 339 [M+H-146-18-18-28]+ | 12-hydroxy-calactin a 2311818-49-0 | Latex, flowers, leaves, branches, roots | standard |
| 3 | C29H40O10 | 549.2622 | 531 [M+H-18]+ 513 [M+H-18-18]+ 485 [M+H-18-18-28]+ 467 [M+H-18-18-28-18]+ 385 [M+H-146-18]+ 367 [M+H-146-18-18]+ 339 [M+H-146-18-18-28]+ | 15-hydroxy-calactin a 159406-83-4 | Latex, flowers, leaves, branches, roots | standard |
| 4 | C23H32O6 | 405.2254 | 387 [M+H-18]+ 369 [M+H-18-18]+ 341 [M+H-18-18-28]+ 323 [M+H-18-18-28-18]+ | Calotropageninb 24211-64-1 | Latex, flowers, leaves, branches, roots | [34,35,36,37,38,39] |
| 5 | C23H34O6 | 407. 2411 | 389 [M+H-18]+ 371 [M+H-18-18]+ 353 [M+H-18-18-18]+ | Hydroxy coroglaucigenin isomer b tentatively identified | Latex, flowers, leaves, branches, roots | [40] |
| 6 | C29H40O10 | 549.2622 | 531 [M+H-18]+ 513 [M+H-18-18]+ 485 [M+H-18-18-28]+ 467 [M+H-18-18-28-18]+ 385 [M+H-146-18]+ 367 [M+H-146-18-18]+ 339 [M+H-146-18-18-28]+ | 16-hydroxy-calactin a 107110-13-4 | Latex, flowers, leaves, branches, roots | standard |
| 7 | C35H54O14 | 699.3617 | 537 [M+H-162]+ 375 [M+H-162]+ | Uzarin b 20231-81-6 | Latex, flowers, leaves, branches, roots | [41] |
| 8 | C29H40O10 | 549.2622 | 531 [M+H-18]+ 513 [M+H-18-18]+ 485 [M+H-18-18-28]+ 467 [M+H-18-18-28-18]+ 387 [M+H-162]+ 369 [M+H-162-18]+ 351 [M+H-162-18-18]+ 323 [M+H-162-18-18-28]+ | Calotoxin b 20304-49-8P | Latex, flowers, leaves, branches, roots | [34,35,38,39,40,42,43,44] |
| 9 | C29H44O9 | 537.3037 | 519 [M+H-18]+ 501 [M+H-18-18]+ 391 [M+H-146]+ 373 [M+H-146-18]+ 355 [M+H-146-18-18]+ | Frugoside b 546-02-1 | Latex, flowers, leaves, branches, roots | [36,45,46] |
| 10 | C29H40O10 | 549.2622 | 531 [M+H-18]+ 513 [M+H-18-18]+ 485 [M+H-18-18-28]+ 467 [M+H-18-18-28-18]+ 405 [M+H-144]+ 387 [M+H-144-18]+ | Calactinic acid b 24321-45-7 | Latex, flowers, leaves, branches, roots | [47] |
| 11 | C23H34O5 | 391.2464 | 373 [M+H-18]+ 355 [M+H-18-18]+ 337 [M+H-18-18-18]+ | Coroglaucigenin b 468-19-9 | Latex, flowers, leaves, branches, roots | [36,37] |
| 12 | C29H44O9 | 537.3037 | 519 [M+H-18]+ 501 [M+H-18-18]+ 375 [M+H-162]+ 357 [M+H-162-18]+ 339 [M+H-162-18-18]+ | Desglucouzarin b 6877-82-3P | Latex, flowers, leaves, branches | [45] |
| 13 | C29H42O9 | 535.2901 | 517 [M+H-18]+ 499 [M+H-18-18]+ 389 [M+H-146]+ 371 [M+H-146-18]+ 353 [M+H-146-18-18]+ | Afroside b 29010-26-2P | Latex, flowers, leaves, branches, roots | [40] |
| 14 | C31H41NO9S | 604.2544 | 586 [M+H-18]+ 568 [M+H-18-18]+ 403 [M+H-201]+ 385 [M+H-201-18]+ | 15-hydroxy uscharin b 29010-26-2 | Latex, flowers, leaves, branches, roots | [40] |
| 15 | C35H54O13 | 683.3630 | 521[M+H-162]+ 375 [M+H-162-146]+ 357 [M+H-162-146-18]+ 339 [M+H-162-146-18-18]+ | Calotropisprocerasaponin I b tentatively identified | Latex, flowers, leaves, branches, roots | [41] |
| 16 | C29H40O9 | 533.2745 | 515 [M+H-18]+ 497 [M+H-18-18-28]+ 387 [M+H-146]+ 369 [M+H-146-18]+ 351 [M+H-146-18-18]+ 323 [M+H-146-18-18-28]+ | Calactin a 20304-47-6 | Latex, flowers, leaves, branches, roots | [34,35,38,39,42,43,44,48] |
| 17 | C29H40O10 | 563.2837 | 545 [M+H-18]+ 513 [M+H-32]+ 387 [M+H-176]+ 369 [M+H-176-18]+ 351 [M+H-176-18-18]+ 323 [M+H-176-18-18- 28]+ | Calactinic acid methylester b 24211-77-6 | Latex, flowers, leaves, branches, roots | [38] |
| 18 | C29H40O9 | 533.2745 | 515 [M+H-18]+ 497 [M+H-18-18]+ 497 [M+H-18-18-28]+ 387 [M+H-146]+ 369 [M+H-146-18]+ 351 [M+H-146-18-18]+ 323 [M+H-146-18-18-28]+ | Calotropin a 1986-70-5P | Latex, flowers, leaves, branches, roots | [34,35,36,39,42,43,44,46,48] |
| 19 | C31H43NO8S | 590.2759 | 572 [M+H-18]+ 554 [M+H-18-18]+ 526 [M+H-18-18-28]+ 387 [M+H-203]+ 369 [M+H-203-18]+ 351 [M+H-201-18-18]+ 323 [M+H-201-18-18-28]+ | Voruscharin b 27892-03-1 | Latex | [34,35,39,42,43,44,48] |
| 20 | C23H34O4 | 375.2519 | 357 [M+H-18]+ 339 [M+H-18-18]+ 321 [M+H-18-18-18]+ | Uzarigenin b 466-09-1P | Latex, flowers, leaves, branches, roots | [34,36,41,42,49] |
| 21 | C31H41NO9S | 604.2544 | 586 [M+H-18]+ 568 [M+H-18-18]+ 540 [M+H-28-18-18]+ 387 [M+H-217]+ 369 [M+H-217-18]+ 351 [M+H-217-18-18]+ 323 [M+H-217-18-18-28]+ | 2″-Oxovoruscharin b 676541-57-4 | Latex, roots | [42,48] |
| 22 | 636.2454 | 618 [M+H-18]+ 387 [M+H-247]+ 369 [M+ H-247-18]+ 351 [M+ H-247-18-18]+ 323 [M+ H-247-18-18-28]+ | Calotropagenin glycoside I b tentatively identified | Latex, branches, roots | [38] | |
| 23 | C31H42O10 | 575.2828 | 557 [M+H-18]+ 539 [M+H-18-18]+ 497 [M+H-42-18-18]+ | Asclepin b 36573-63-4 | Latex, flowers, leaves, branches, roots | [36,38] |
| 24 | C29H38O9 | 531.2589 | 513 [M+H-18]+ 485 [M+H-18-18]+ 467 [M+H-18-18-18]+ 387 [M+H-144]+ 369 [M+H-144-18]+ 351 [M+H-144-18-18]+ 323 [M+H-144-18-18-28]+ | Uscharidin b 24211-81-2P | Latex, flowers, leaves, branches, roots | [34,36,38,39,43,44] |
| 25 | C31H43NO8S | 590.2759 | 572 [M+H-18]+ 554 [M+H-18-18]+ 526 [M+H-18-18-28]+ 389 [M+H-201]+ 371 [M+ H-201-18]+ 353 [M+ H-201-18-18]+ 325 [M+ H-201-18-18-28]+ | Labriformine b 66419-07-6 | Latex | [39] |
| 26 | 634.2667 | 618 [M+H-18]+ 556 [M+H-18-18]+ 538 [M+H-18-18-28]+ 387 [M+H-215]+ 369 [M+ H-215-18]+ 351 [M+ H-215-18-18]+ 323 [M+ H-215-18-18-28]+ | Calotropagenin glycoside III b tentatively identified | Latex, flowers, leaves, branches, roots | [38] | |
| 27 | C31H41NO8S | 588.2597 | 570 [M+H-18]+ 552 [M+H-18-18]+ 524 [M+H-28-18-18]+ 387 [M+H-201]+ 369 [M+H-201-18]+ 351 [M+H-201-18-18]+ 323 [M+H-201-18-18-28]+ | Uscharin a 24211-81-2 | Latex, leaves | [34,35,36,39,40,42,43,44,48] |
| 28 | 648.2819 | 387 [M+H-261]+ 369 [M+ H-261-18]+ 351 [M+ H-261-18-18]+ 323 [M+ H-261-18-18-28]+ | Calotropagenin glycoside IV b tentatively identified | Latex | [38] | |
| 29 | 602.2391 | 584 [M+H-18]+ 566 [M+H-80]+ 387 [M+H-249]+ 369 [M+ H-249-18]+ 351 [M+ H-249-18-18]+ 323 [M+ H-249-18-18-28]+ | Calotropagenin glycoside II b tentatively identified | Latex | [38] | |
| Flavonoids | ||||||
| 30 | C27H30O16 | 611.1607 | 465 [M+H-146]+ 303 [M+H-146-162]+ | Rutin a 153-18-4 | Flowers, leaves, branches, roots | [50] |
| 31 | C27H30O16 | 611.1607 | 479 [M+H-132]+ 317 [M+H-132-162]+ | Isorhamnetin-hexoside-pentoside b tentatively identified | Flowers, leaves | MS data |
| 32 | C27H30O15 | 595.1657 | 449 [M+H-146]+ 287 [M+H-146-162]+ | Kaempferol-robinoside a 17297-56-2 | Flowers, leaves, branches, roots | standard |
| 33 | C28H32O16 | 625.1763 | 479 [M+H-146]+ 317 [M+H-146-162]+ | Isorhamnetin-robinoside b 107740-46-5 | Flowers, leaves, branches, roots | [42] |
| 34 | C27H30O15 | 595.16575 | 449 [M+H-146]+ 287 [M+H-146-162]+ | Kaempferol-rutinoside a 17650-84-9 | Flowers, leaves, branches, roots | standard |
| 35 | C28H32O16 | 625.1763 | 479 [M+H-146]+ 317 [M+H-146-162]+ | Isorhamnetin-rutinoside b 604-80-8 | Flowers, leaves, branches, roots | [42] |
| 36 | C22H22O12 | 479.1171 | 317 [M+H-162]+ | Isorhamnetin-Hexoside b 1456622-02-8 | Flowers, leaves, branches, roots | MS data |
| 37 | C21H20O11 | 449.1066 | 287 [M+H-162]+ | Kaempferol-Hexoside a 1108717-10-7 | Flowers, leaves, roots | MS data |
| Compound | MRM Transition | LloQ (ng/mL) | Accuracy (%) | Precision (%) | Linearity (0–24 µg/mL) |
|---|---|---|---|---|---|
| Calactin | 533/323 | 25 | 91 | 88 | 0.9987 |
| 15-Hydroxy calactin | 549/199 | 25 | 85 | 81 | 0.9991 |
| Rutin | 611/303 | 10 | 92 | 85 | 0.9976 |
| Isorhamnetin glycoside | 625/317 | 100 | 90 | 87 | 0.9977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bader, A.; Omran, Z.; Al-Asmari, A.I.; Santoro, V.; De Tommasi, N.; D’Ambola, M.; Dal Piaz, F.; Conti, B.; Bedini, S.; Halwani, M. Systematic Phytochemical Screening of Different Organs of Calotropis procera and the Ovicidal Effect of Their Extracts to the Foodstuff Pest Cadra cautella. Molecules 2021, 26, 905. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040905
Bader A, Omran Z, Al-Asmari AI, Santoro V, De Tommasi N, D’Ambola M, Dal Piaz F, Conti B, Bedini S, Halwani M. Systematic Phytochemical Screening of Different Organs of Calotropis procera and the Ovicidal Effect of Their Extracts to the Foodstuff Pest Cadra cautella. Molecules. 2021; 26(4):905. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040905
Chicago/Turabian StyleBader, Ammar, Ziad Omran, Ahmed I. Al-Asmari, Valentina Santoro, Nunziatina De Tommasi, Massimiliano D’Ambola, Fabrizio Dal Piaz, Barbara Conti, Stefano Bedini, and Majed Halwani. 2021. "Systematic Phytochemical Screening of Different Organs of Calotropis procera and the Ovicidal Effect of Their Extracts to the Foodstuff Pest Cadra cautella" Molecules 26, no. 4: 905. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040905