Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.1.1. Ligand Synthesis
2.1.2. Copper(I) Complexes Synthesis
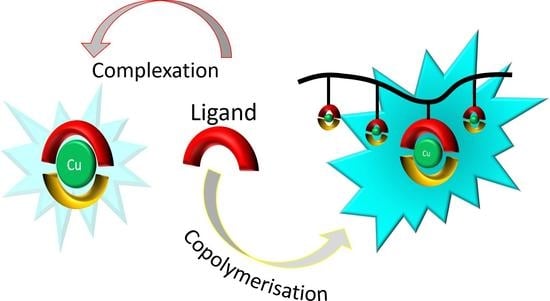
2.2. Synthesis and Structural Characterisation of Metallopolymers with Cu(I) Complexes
2.3. Photophysical Characterisation of the Cu(I) Complexes
2.3.1. In Solution
| Sample | λabs (nm) | ε ×10−3 (M−1 cm−1) | λem a (nm) | Φ b | τ (ns) c |
|---|---|---|---|---|---|
| 5a | 347 276 250 | 4.3 23.5 27.3 | 552 | n.a. | 850 |
| 5b | 347 276 250 | 4.4 22.7 28.5 | 552 | 0.037 | 1050 |
| 5c | 354 273 250 | 4.6 43.0 38.6 | 548 | 0.009 | 83 |
| 5d | 347 281 240 | 3.6 22.0 30.7 | 545 | 0.035 | 1500 |
2.3.2. In Solid State
2.4. Computational Studies of Cu(I) Complexes
2.5. Electrochemical Properties of Cu(I) Complexes
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.2.1. 2-(2-Propyn-1-ol)-6-methylpyridine (2a) and 2-(3-Butynyl-1-ol)-6-methylpyridine (2b)
3.2.2. (1-Benzyl-4-(6-methylpyridin-2-yl)-1H-1,2,3-triazol-5yl)methanol (3a) and 2-(1-Benzyl-5-(6-methylpyridin-2-yl)-1H-1,2,3-triazol-4-yl)ethan-1-ol (3b)
3.2.3. 2-(1-Benzyl-4-(6-methylpyridin-2-yl)-1H-1,2,3-triazol-5-yl)ethyl 4-vinylbenzoate (4c)
3.2.4. 2-(((2-(1-benzyl-4-(6-methylpyridin-2-yl)-1H-1,2,3-triazol-5-yl)ethoxy)carbonyl)amino)ethyl acrylate (4d)
3.2.5. Heteroleptic Cu(I) Complexes 5a, 5b, 5c, 5d
3.2.6. Synthesis of Poly(methyl acrylate) Homopolymer PMA
3.2.7. Synthesis of Poly(styrene) Homopolymer PS
3.2.8. Synthesis of Poly(Sty-co-4c): Copolymer 6c
3.2.9. Synthesis of Poly(MA-co-4d): Copolymer 6d
3.2.10. Post-Polymerisation Complexation to Obtain Functional Copolymers 7c and 7d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yam, V.W.; Au, V.K.; Leung, S.Y. Light-emitting self-assembled materials based on d(8) and d(10) transition metal complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.-W.; Wong, K.M.-C. Luminescent metal complexes of d6, d8 and d10 transition metal centres. Chem. Commun. 2011, 47, 11579–11592. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Demas, J.N.; DeGraff, B.A. Applications of luminescent transition platinum group metal complexes to sensor technology and molecular probes. Coord. Chem. Rev. 2001, 211, 317–351. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.B.; Wenger, O.S. Photoredox catalysis with metal complexes made from earth-abundant elements. Chem. Eur. J. 2018, 24, 2039–2058. [Google Scholar] [CrossRef] [Green Version]
- Busch, J.; Knoll, D.M.; Zippel, C.; Bräse, S.; Bizzarri, C. Metal-supported and -assisted stereoselective cooperative photoredox catalysis. Dalton Trans. 2019, 48, 15338–15357. [Google Scholar] [CrossRef] [Green Version]
- Volz, D.; Wallesch, M.; Fléchon, C.; Danz, M.; Verma, A.; Navarro, J.M.; Zink, D.M.; Bräse, S.; Baumann, T. From iridium and platinum to copper and carbon: New avenues for more sustainability in organic light-emitting diodes. Green Chem. 2015, 17, 1988–2011. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Iwamura, M.; Takeuchi, S.; Tahara, T. Real-Time Observation of the Photoinduced Structural Change of Bis(2,9-dimethyl-1,10-phenanthroline)copper(I) by Femtosecond Fluorescence Spectroscopy: A Realistic Potential Curve of the Jahn−Teller Distortion. J. Am. Chem. Soc. 2007, 129, 5248–5256. [Google Scholar] [CrossRef]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent complexes beyond the platinum group: The d10 avenue. Chem. Commun. 2008, 19, 2185–2193. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and photophysics of coordination compounds: Copper. In Photochemistry and Photophysics of Coordination Compounds I; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–115. [Google Scholar]
- Garakyaraghi, S.; McCusker, C.E.; Khan, S.; Koutnik, P.; Bui, A.T.; Castellano, F.N. Enhancing the visible-light absorption and excited-state properties of Cu(I) MLCT excited states. Inorg. Chem. 2018, 57, 2296–2307. [Google Scholar] [CrossRef]
- Garakyaraghi, S.; Danilov, E.O.; McCusker, C.E.; Castellano, F.N. Transient absorption dynamics of sterically congested Cu(I) MLCT excited states. J. Phys. Chem. A 2015, 119, 3181–3193. [Google Scholar] [CrossRef]
- Kuang, S.-M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and structural characterization of Cu(I) and Ni(II) complexes that contain the Bis[2-(diphenylphosphino)phenyl]ether ligand. Novel emission properties for the Cu(I) species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar] [CrossRef]
- Kaeser, A.; Mohankumar, M.; Mohanraj, J.; Monti, F.; Holler, M.; Cid, J.-J.; Moudam, O.; Nierengarten, I.; Karmazin-Brelot, L.; Duhayon, C.; et al. Heteroleptic copper(I) complexes prepared from phenanthroline and bis-phosphine ligands. Inorg. Chem. 2013, 52, 12140–12151. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.i.; Armaroli, N. Heteroleptic copper(I) complexes prepared from phenanthroline and bis-phosphine ligands: Rationalization of the photophysical and electrochemical properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Bizzarri, C.; Hundemer, F.; Busch, J.; Bräse, S. Triplet emitters versus TADF emitters in OLEDs: A comparative study. Polyhedron 2018, 140, 51–66. [Google Scholar] [CrossRef]
- Ravaro, L.P.; Zanoni, K.P.S.; de Camargo, A.S.S. Luminescent copper(I) complexes as promising materials for the next generation of energy-saving OLED devices. Energy Rep. 2020, 6, 37–45. [Google Scholar] [CrossRef]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th Anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef]
- Neumann, L.N.; Urban, D.A.; Lemal, P.; Ramani, S.; Petri-Fink, A.; Balog, S.; Weder, C.; Schrettl, S. Preparation of metallosupramolecular single-chain polymeric nanoparticles and their characterization by Taylor dispersion. Polym. Chem. 2020, 11, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Willenbacher, J.; Altintas, O.; Roesky, P.W.; Barner-Kowollik, C. Single-chain self-folding of synthetic polymers induced by metal–ligand complexation. Macromol. Rapid Commun. 2014, 35, 45–51. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Arbe, A.; Colmenero, J.; Pomposo, J.A. Metallo-folded single-chain nanoparticles with catalytic selectivity. ACS Macro Lett. 2014, 3, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Astruc, D.; Abd-El-Aziz, A.S. Metallopolymers for advanced sustainable applications. Chem. Soc. Rev. 2019, 48, 558–636. [Google Scholar] [CrossRef] [PubMed]
- Volz, D.; Hirschbiel, A.F.; Zink, D.M.; Friedrichs, J.; Nieger, M.; Baumann, T.; Bräse, S.; Barner-Kowollik, C. Highly efficient photoluminescent Cu(i)–PyrPHOS-metallopolymers. J. Mater. Chem. C 2014, 2, 1457–1462. [Google Scholar] [CrossRef]
- Elmas, S.; Macdonald, T.J.; Skinner, W.; Andersson, M.; Nann, T. Copper metallopolymer catalyst for the electrocatalytic hydrogen evolution reaction (HER). Polymers 2019, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- De Hatten, X.; Bell, N.; Yufa, N.; Christmann, G.; Nitschke, J.R. A Dynamic covalent, luminescent metallopolymer that undergoes sol-to-gel transition on temperature rise. J. Am. Chem. Soc. 2011, 133, 3158–3164. [Google Scholar] [CrossRef]
- Greenfield, J.L.; Rizzuto, F.J.; Goldberga, I.; Nitschke, J.R. Self-assembly of conjugated metallopolymers with tunable length and controlled regiochemistry. Angew. Chem. Int. Ed. 2017, 56, 7541–7545. [Google Scholar] [CrossRef]
- Asil, D.; Foster, J.A.; Patra, A.; de Hatten, X.; del Barrio, J.; Scherman, O.A.; Nitschke, J.R.; Friend, R.H. Temperature- and Voltage-Induced Ligand Rearrangement of a Dynamic Electroluminescent Metallopolymer. Angew. Chem. Int. Ed. 2014, 53, 8388–8391. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Johansson, J.R.; Beke-Somfai, T.; Said Stalsmeden, A.; Kann, N. Ruthenium-catalyzed azide alkyne cycloaddition reaction: Scope, mechanism, and applications. Chem. Rev. 2016, 116, 14726–14768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic Azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef] [PubMed]
- Neises, B.; Steglich, W. Simple Method for the esterification of carboxylic acids. Angew. Chem. Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Jones, P.G.; Crespo, O. Tetrakis(acetonitrile-N)copper(I) tetrafluoroborate. Acta Crystallogr. Sect. C 1998, 54, 18–20. [Google Scholar] [CrossRef]
- Bizzarri, C.; Arndt, A.P.; Kohaut, S.; Fink, K.; Nieger, M. Mononuclear and dinuclear heteroleptic Cu(I) complexes based on pyridyl-triazole and DPEPhos with long-lived excited-state lifetimes. J. Organomet. Chem. 2018, 871, 140–149. [Google Scholar] [CrossRef]
- Sun, Y.; Lemaur, V.; Beltran, J.I.; Cornil, J.; Huang, J.; Zhu, J.; Wang, Y.; Frohlich, R.; Wang, H.; Jiang, L.; et al. Neutral mononuclear copper(I) complexes: Synthesis, crystal structures, and photophysical properties. Inorg. Chem. 2016, 55, 5845–5852. [Google Scholar] [CrossRef]
- Shields, G.P.; Raithby, P.R.; Allen, F.H.; Motherwell, W.D.S. The assignment and validation of metal oxidation states in the Cambridge Structural Database. Acta Crystallogr. Sect. B 2000, 56, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyjaszewski, K.; Woodworth, B.E. Interaction of propagating radicals with copper(I) and copper(II) species. Macromolecules 1998, 31, 4718–4723. [Google Scholar] [CrossRef]
- Gernert, M.; Balles-Wolf, L.; Kerner, F.; Müller, U.; Schmiedel, A.; Holzapfel, M.; Marian, C.M.; Pflaum, J.; Lambert, C.; Steffen, A. Cyclic (Amino)(aryl)carbenes enter the field of chromophore ligands: Expanded π system leads to unusually deep red emitting CuI compounds. J. Am. Chem. Soc. 2020, 142, 8897–8909. [Google Scholar] [CrossRef] [PubMed]
- Gernert, M.; Muller, U.; Haehnel, M.; Pflaum, J.; Steffen, A. A Cyclic Alkyl(amino)carbene as two-atom pi-chromophore leading to the first phosphorescent linear Cu(I) complexes. Chemistry 2017, 23, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- So, G.K.; Cheng, G.; Wang, J.; Chang, X.; Kwok, C.C.; Zhang, H.; Che, C.M. Efficient color-tunable copper(I) complexes and their applications in solution-processed organic light-emitting diodes. Chem. Asian J. 2017, 12, 1490–1498. [Google Scholar] [CrossRef] [Green Version]
- Wallesch, M.; Verma, A.; Flechon, C.; Flugge, H.; Zink, D.M.; Seifermann, S.M.; Navarro, J.M.; Vitova, T.; Gottlicher, J.; Steininger, R.; et al. Towards printed organic light-emitting devices: A solution-stable, highly soluble Cu(I) -NHetPHOS. Chemistry 2016, 22, 16400–16405. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Verma, A.; Zink, D.M.; Fléchon, C.; Leganés Carballo, J.; Flügge, H.; Navarro, J.M.; Baumann, T.; Volz, D. Efficient, inkjet-printed TADF-OLEDs with an ultra-soluble NHetPHOS complex. Appl. Phys. A 2016, 122, 1–5. [Google Scholar] [CrossRef]
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) complexes for thermally activated delayed fluorescence: From photophysical to device properties. Top. Curr. Chem. 2016, 374, 25. [Google Scholar] [CrossRef]
- Osawa, M.; Hoshino, M.; Hashimoto, M.; Kawata, I.; Igawa, S.; Yashima, M. Application of three-coordinate copper(I) complexes with halide ligands in organic light-emitting diodes that exhibit delayed fluorescence. Dalton Trans. 2015, 44, 8369–8378. [Google Scholar] [CrossRef] [PubMed]
- Leitl, M.J.; Kuchle, F.R.; Mayer, H.A.; Wesemann, L.; Yersin, H. Brightly blue and green emitting Cu(I) dimers for singlet harvesting in OLEDs. J. Phys. Chem. A 2013, 117, 11823–11836. [Google Scholar] [CrossRef]
- Busch, J.M.; Zink, D.M.; Di Martino-Fumo, P.; Rehak, F.R.; Boden, P.; Steiger, S.; Fuhr, O.; Nieger, M.; Klopper, W.; Gerhards, M.; et al. Highly soluble fluorine containing Cu(i) AlkylPyrPhos TADF complexes. Dalton Trans. 2019, 48, 15687–15698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huitorel, B.; El Moll, H.; Utrera-Melero, R.; Cordier, M.; Fargues, A.; Garcia, A.; Massuyeau, F.; Martineau-Corcos, C.; Fayon, F.; Rakhmatullin, A.; et al. Evaluation of ligands effect on the photophysical properties of copper iodide clusters. Inorg. Chem. 2018, 57, 4328–4339. [Google Scholar] [CrossRef]
- El Sayed Moussa, M.; Khalil, A.M.; Evariste, S.; Wong, H.-L.; Delmas, V.; Le Guennic, B.; Calvez, G.; Costuas, K.; Yam, V.W.-W.; Lescop, C. Intramolecular rearrangements guided by adaptive coordination-driven reactions toward highly luminescent polynuclear Cu(i) assemblies. Inorg. Chem. Front. 2020, 7, 1334–1344. [Google Scholar] [CrossRef]
- Evariste, S.; Khalil, A.M.; Moussa, M.E.; Chan, A.K.-W.; Hong, E.Y.-H.; Wong, H.-L.; Le Guennic, B.; Calvez, G.; Costuas, K.; Yam, V.W.-W.; et al. Adaptive coordination-driven supramolecular syntheses toward new polymetallic Cu(I) luminescent assemblies. J. Am. Chem. Soc. 2018, 140, 12521–12526. [Google Scholar] [CrossRef] [PubMed]
- Chakkaradhari, G.; Eskelinen, T.; Degbe, C.; Belyaev, A.; Melnikov, A.S.; Grachova, E.V.; Tunik, S.P.; Hirva, P.; Koshevoy, I.O. Oligophosphine-thiocyanate Copper(I) and Silver(I) complexes and their borane derivatives showing delayed fluorescence. Inorg. Chem. 2019, 58, 3646–3660. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Nobuyasu, R.S.; Zhang, B.; Geng, Y.; Yao, B.; Xie, Z.; Zhu, D.; Shan, G.; Che, W.; Yan, L.; et al. Thermally activated delayed fluorescence in CuI complexes originating from restricted molecular vibrations. Chem. Eur. J. 2017, 23, 11761–11766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chen, J.; Wu, X.Y.; Chen, X.L.; Yu, R.; Lu, C.Z. Outstanding blue delayed fluorescence and significant processing stability of cuprous complexes with functional pyridine-pyrazolate diimine ligands. Dalton Trans. 2015, 44, 6706–6710. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; La Placa, M.-G.; Junquera-Hernández, J.M.; Bolink, H.J.; Constable, E.C.; Sessolo, M.; Ortí, E.; Housecroft, C.E. The shiny side of copper: Bringing copper(i) light-emitting electrochemical cells closer to application. RSC Adv. 2020, 10, 22631–22644. [Google Scholar] [CrossRef]
- Brunner, F.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Positional isomerism in the N^N ligand: How much difference does a methyl group make in [Cu(P^P)(N^N)](+) complexes? Molecules 2020, 25, 2760. [Google Scholar] [CrossRef]
- Ishida, H.; Bünzli, J.-C.; Beeby, A. Guidelines for measurement of luminescence spectra and quantum yields of inorganic and organometallic compounds in solution and solid state (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Fries, F.; Reineke, S. Statistical treatment of photoluminescence quantum yield measurements. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.A. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley Inc.: New York, NY, USA, 2000. [Google Scholar]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis(Acetonitrile)Copper(I) Hexafluorophosphate. In Inorganic Syntheses, 2nd ed.; Shriver, D.F., Ed.; Inorganic Syntheses Organization, John Wiley & Sons: Toronto, ON, Canada, 1979; p. 90. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Polymer | Mn (kg/mol) | Đ |
|---|---|---|
| 6c | 13.5 | 1.65 |
| 7c | 11.8 | 1.63 |
| 6d | 9.54 | 1.92 |
| 7d | 7.26 | 1.62 |
| Sample | λem (nm) | Φ a |
|---|---|---|
| 5a | 511 | 0.06 (±0.02) |
| 5b | 504 | 0.12 (±0.02) |
| 5c | 501 | 0.09 b (±0.12) |
| 5d | 503 | 0.17 (±0.01) |
| 7c | 508 | 0.09 (±0.01) |
| 7d | 503 | 0.10 (±0.01) |
| PS/5c | n.d. | n.d. |
| PMA/5c | n.d. | n.d. |
| PS/5d | 502 | 0.13 (±0.02) |
| PMA/5d | 507 | 0.21 (±0.01) |
| Sample | Eox/V | Ered1/V | Ered2/V | ΔHOMO-LUMO/eV | ΔHOMO-LUMO c/eV |
|---|---|---|---|---|---|
| 5a | n.a.; 0.84 b | n.a. | n.a. | n.a. | n.a. |
| 5b | 0.68; 0.82 b | −2.47 | −2.84 | 3.15 | 4.35 |
| 5c | 0.68; 0.84 b | −2.33 | −2.70 | 3.01 | 4.17 |
| 5d | 0.64; 0.88 b | −2.48 | −2.94 | 3.12 | 4.37 |
| Compound | Alcohol | Quantity (g) | Yield (%) |
|---|---|---|---|
| 2a | 8a | 0.98 | 71.3 |
| 2b | 8b | 1.22 | 72.5 |
| Compound | Yield (%) |
|---|---|
| 3a | 52.3 |
| 3b | 64.3 |
| Product | Yield (%) |
|---|---|
| 5a | 63.6 |
| 5b | 68.3 |
| 5c | 59.2 |
| 5d | 65.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.; Braun, J.; Anson, C.E.; Wilts, B.D.; Moatsou, D.; Bizzarri, C. Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers. Molecules 2021, 26, 2567. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092567
Ferrari F, Braun J, Anson CE, Wilts BD, Moatsou D, Bizzarri C. Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers. Molecules. 2021; 26(9):2567. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092567
Chicago/Turabian StyleFerrari, Federico, Jonas Braun, Christopher E. Anson, Bodo D. Wilts, Dafni Moatsou, and Claudia Bizzarri. 2021. "Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers" Molecules 26, no. 9: 2567. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092567