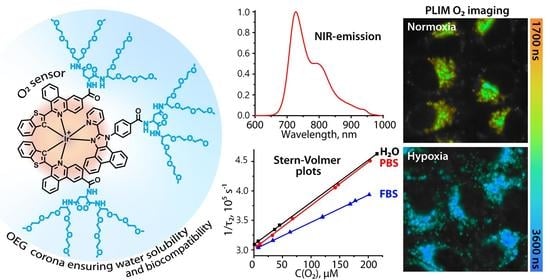
Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Photophysical Properties
3.2. Biological Experiments
3.2.1. Cytotoxicity Tests
- (i)
- Ir1 displays higher toxicity compared to its relatives (Ir2 and Ir2a) containing branched OEG pendants;
- (ii)
- Incubation of cancer (CT26 and HeLa) and normal (CHO-K1) cells with Ir1 at the concentrations above 25 μM reduced cell viability by >20%;
- (iii)
- For Ir2 comparable level of toxicity was not achieved even at the concentration 150 μM;
- (iv)
- Ir2a is only slightly more toxic than Ir2.

3.2.2. Dynamics of the Complexes’ Uptake.
3.2.3. Colocalization Experiments.
3.2.4. PLIM Experiments
4. Materials and Methods
4.1. Synthesis of Ligands and Complexes
4.2. Photophysical Experiments
4.3. Cell Cultures
4.4. MTT Assay
4.5. Cell Compartment Staining
4.6. Confocal Microscopy and Colocalization Assay
4.7. PLIM
4.8. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsai, A.G.; Johnson, P.C.; Intaglietta, M. Oxygen gradients in the microcirculation. Physiol. Rev. 2003, 83, 933–963. [Google Scholar] [CrossRef] [Green Version]
- Masson, N.; Ratcliffe, P.J. Hypoxia signaling pathways in cancer metabolism: The importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-modified cancer cell metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular mechanisms of ischemia–reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Ham, P.B.; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2017, 157, 92–116. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Galloway, N.R.; Amoaku, W.M.K.; Galloway, P.H.; Browning, A.C. (Eds.) Basic anatomy and physiology of the eye. In Common Eye Diseases and their Management; Springer: London, UK, 2006; pp. 7–15. ISBN 978-1-84628-033-7. [Google Scholar]
- Holekamp, N.M.; Shui, Y.B.; Beebe, D.C. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 2005, 139, 302–310. [Google Scholar] [CrossRef]
- Borisov, S.M. Fundamentals of Quenched Phosphorescence O2 Sensing and Rational Design of Sensor Materials. In Quenched-phosphorescence Detection of Molecular Oxygen: Applications in Life Sciences; The Royal Society of Chemistry: London, UK, 2018; pp. 1–18. ISBN 978-1-78801-175-4. [Google Scholar]
- Mycek, M.A.; Pogue, B.W. Handbook of Biomedical Fluorescence; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9781135542580. [Google Scholar]
- Felder, A.E.; Wanek, J.; Tan, M.R.; Blair, N.P.; Shahidi, M. A method for combined retinal vascular and tissue oxygen tension imaging. Sci. Rep. 2017, 7, 10622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezu-Ndubuisi, O.J.; Teng, P.; Wanek, J.; Blair, N.P.; Chau, F.Y.; Reddy, N.M.; Raj, J.U.; Reddy, S.P.; Shahidi, M. In vivo retinal vascular oxygen tension imaging and fluorescein angiography in the mouse model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6968–6972. [Google Scholar] [CrossRef]
- Yoshihara, T.; Karasawa, Y.; Zhang, S.; Hosaka, M.; Takeuchi, T.; Iida, Y.; Endo, K.; Imamura, T.; Tobita, S. In vivo phosphorescence imaging of cancer using iridium complexes. In Proceedings of the Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications; Achilefu, S., Raghavachari, R., Eds.; SPIE BiOS: San Jose, CA, USA, 2009; Volume 7190, p. 71900X. [Google Scholar]
- Carraway, E.R.; Demas, J.N.; DeGraff, B.A.; Bacon, J.R. Photophysics and photochemistry of oxygen sensors based on luminescent transition-metal complexes. Anal. Chem. 1991, 63, 337–342. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Time-resolved emission imaging microscopy using phosphorescent metal complexes: Taking flim and plim to new lengths. In Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Lo, K.K.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 205–256. ISBN 978-3-662-46718-3. [Google Scholar]
- Jenkins, J.; Dmitriev, R.I.; Papkovsky, D.B. Imaging cell and tissue O2 by TCSPC-PLIM. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 225–247. ISBN 978-3-319-14929-5. [Google Scholar]
- Becker, W.; Shcheslavskiy, V.; Rück, A. Simultaneous phosphorescence and fluorescence lifetime imaging by multi-dimensional tcspc and multi-pulse excitation. In Multi-Parametric Live Cell Microscopy of 3D Tissue Models; Dmitriev, R.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–30. ISBN 978-3-319-67358-5. [Google Scholar]
- Sud, D.; Mycek, M.A. Calibration and validation of an optical sensor for intracellular oxygen measurements. J. Biomed. Opt. 2009, 14, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papkovsky, D.B.; Dmitriev, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Pan, T.; Chen, L.; Tian, Y.; Zhang, W. Luminescence materials for pH and oxygen sensing in microbial cells–structures, optical properties, and biological applications. Crit. Rev. Biotechnol. 2017, 37, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Solomatina, A.I.; Su, S.H.; Lukina, M.M.; Dudenkova, V.V.; Shcheslavskiy, V.I.; Wu, C.H.; Chelushkin, P.S.; Chou, P.T.; Koshevoy, I.O.; Tunik, S.P. Water-soluble cyclometalated platinum(II) and iridium(III) complexes: Synthesis, tuning of the photophysical properties, and in vitro and in vivo phosphorescence lifetime imaging. RSC Adv. 2018, 8, 17224–17236. [Google Scholar] [CrossRef] [Green Version]
- Shcheslavskiy, V.I.; Neubauer, A.; Bukowiecki, R.; Dinter, F.; Becker, W. Combined fluorescence and phosphorescence lifetime imaging. Appl. Phys. Lett. 2016, 108, 91111. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Guo, S.; Jiang, J.; Zhang, P.; Han, J.; Ma, Y.; Zhao, Q. Anionic iridium(III) complexes and their conjugated polymer soft salts for time-resolved luminescent detection of intracellular oxygen levels. Sensors Actuators B Chem. 2018, 262, 436–443. [Google Scholar] [CrossRef]
- Shewring, J.R.; Cankut, A.J.; McKenzie, L.K.; Crowston, B.J.; Botchway, S.W.; Weinstein, J.A.; Edwards, E.; Ward, M.D. Multimodal probes: Superresolution and transmission electron microscopy imaging of mitochondria, and oxygen mapping of cells, using small-molecule Ir(iii) luminescent complexes. Inorg. Chem. 2017, 56, 15259–15270. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, I.S.; Elistratova, A.A.; Sokolov, V.V.; Chelushkin, P.S.; Shirmanova, M.V.; Lukina, M.M.; Dudenkova, V.V.; Shcheslavskiy, V.I.; Kalinina, S.; Reeß, K.; et al. A biocompatible phosphorescent Ir(iii) oxygen sensor functionalized with oligo(ethylene glycol) groups: Synthesis, photophysics and application in PLIM experiments. New J. Chem. 2020, 44, 10459–10471. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Gao, P.; Sun, G.; Zhang, T.; Li, X.; Liu, S.; Zhao, Q.; Lo, K.K.W.; Huang, W. Dual-phosphorescent Iridium(iii) complexes extending oxygen sensing from hypoxia to hyperoxia. J. Am. Chem. Soc. 2018, 140, 7827–7834. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Bu, W.; Bu, J.; Sun, Y.; Du, J.; Shi, J. Ultrasensitive nanosensors based on upconversion nanoparticles for selective hypoxia imaging in vivo upon near-infrared excitation. J. Am. Chem. Soc. 2014, 136, 9701–9709. [Google Scholar] [CrossRef] [PubMed]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two new “protected” oxyphors for biological oximetry: Properties and application in tumor imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roussakis, E.; Spencer, J.A.; Lin, C.P.; Vinogradov, S.A. Two-photon antenna-core oxygen probe with enhanced performance. Anal. Chem. 2014, 86, 5937–5945. [Google Scholar] [CrossRef]
- Esipova, T.V.; Barrett, M.J.P.; Erlebach, E.; Masunov, A.E.; Weber, B.; Vinogradov, S.A. Oxyphor 2p: A high-performance probe for deep-tissue longitudinal oxygen imaging. Cell Metab. 2019, 29, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fercher, A.; Borisov, S.M.; Zhdanov, A.V.; Klimant, I.; Papkovsky, D.B. Intracellular O2 sensing probe based on cell-penetrating phosphorescent nanoparticles. ACS Nano 2011, 5, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.V.; Dmitriev, R.I.; Borisov, S.M.; Klimant, I.; O’Brien, I.; Nolan, Y.M.; Zhdanov, A.V.; Papkovsky, D.B. A phosphorescent nanoparticle-based probe for sensing and imaging of (intra)cellular oxygen in multiple detection modalities. Adv. Funct. Mater. 2012, 22, 4931–4939. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Borisov, S.M.; Düssmann, H.; Sun, S.; Müller, B.J.; Prehn, J.; Baklaushev, V.P.; Klimant, I.; Papkovsky, D.B. Versatile conjugated polymer nanoparticles for high-resolution O2 imaging in cells and 3D tissue models. ACS Nano 2015, 9, 5275–5288. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Mayr, T.; Mistlberger, G.; Waich, K.; Koren, K.; Chojnacki, P.; Klimant, I. Precipitation as a simple and versatile method for preparation of optical nanochemosensors. Talanta 2009, 79, 1322–1330. [Google Scholar] [CrossRef]
- Deaton, J.C.; Castellano, F.N. Archetypal Iridium(iii) compounds for optoelectronic and photonic applications. In Photophysical Properties and Synthetic Methods; Zysman-Colman, E., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–69. [Google Scholar]
- Zhang, K.Y.; Liu, S.; Zhao, Q.; Li, F.; Huang, W. Phosphorescent Iridium(iii) complexes for bioimaging BT-luminescent and photoactive transition metal complexes as biomolecular probes and cellular reagents. In Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Lo, K.K.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 131–180. ISBN 978-3-662-46718-3. [Google Scholar]
- Che, C.M.; Zhang, J.L.; Lin, L.R. PEG-Linked luminescent platinum(ii) complex as aqueous polymeric molecular light switch for protein binding reactions. Chem. Commun. 2002, 2556–2557. [Google Scholar] [CrossRef]
- Colombo, A.; Fiorini, F.; Septiadi, D.; Dragonetti, C.; Nisic, F.; Valore, A.; Roberto, D.; Mauro, M.; De Cola, L. Neutral N^C^N terdentate luminescent Pt(ii) complexes: Their synthesis, photophysical properties, and bio-imaging applications. Dalt. Trans. 2015, 44, 8478–8487. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, X.; Zou, Y.; Chen, D.; Yang, L.; Rao, J.; Chen, H.; Chan, M.C.W.; Li, L.; Guo, Z.; et al. Mitochondria-targeted platinum(ii) complexes: Dual inhibitory activities on tumor cell proliferation and migration/invasion via intracellular trafficking of β-catenin. Metallomics 2017, 9, 726–733. [Google Scholar] [CrossRef]
- Potocny, A.M.; Riley, R.S.; O’Sullivan, R.K.; Day, E.S.; Rosenthal, J. Photochemotherapeutic properties of a linear tetrapyrrole Palladium(ii) complex displaying an exceptionally high phototoxicity index. Inorg. Chem. 2018, 57, 10608–10615. [Google Scholar] [CrossRef]
- Wang, J.; Potocny, A.M.; Rosenthal, J.; Day, E.S. Gold nanoshell-linear tetrapyrrole conjugates for near infrared-activated dual photodynamic and photothermal therapies. ACS Omega 2020, 5, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lan, M.; To, W.P.; Li, K.; Lok, C.N.; Wang, P.; Che, C.M. A macromolecular cyclometalated gold(iii) amphiphile displays long-lived emissive excited state in water: Self-assembly and in vitro photo-toxicity. Chem. Commun. 2016, 52, 13273–13276. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.M.H.; Lo, K.K.W. Luminescent rhenium(I), ruthenium(II), and iridium(III) polypyridine complexes containing a poly(ethylene glycol) pendant or bioorthogonal reaction group as biological probes and photocytotoxic agents. Coord. Chem. Rev. 2018, 361, 138–163. [Google Scholar] [CrossRef]
- Choi, A.W.T.; Louie, M.W.; Li, S.P.Y.; Liu, H.W.; Chan, B.T.N.; Lam, T.C.Y.; Lin, A.C.C.; Cheng, S.H.; Lo, K.K.W. Emissive behavior, cytotoxic activity, cellular uptake, and pegylation properties of new luminescent rhenium(i) polypyridine poly(ethylene glycol) complexes. Inorg. Chem. 2012, 51, 13289–13302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.Y.; Lau, C.T.S.; Louie, M.W.; Lam, Y.W.; Cheng, S.H.; Lo, K.K.W. Mitochondria-targeting cyclometalated iridium(III)–PEG complexes with tunable photodynamic activity. Biomaterials 2013, 34, 7519–7532. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.W.; Tso, K.K.S. Functionalization of cyclometalated iridium(iii) polypyridine complexes for the design of intracellular sensors, organelle-targeting imaging reagents, and metallodrugs. Inorg. Chem. Front. 2015, 2, 510–524. [Google Scholar] [CrossRef]
- Akiyama, H.; Takahashi, I.; Shimoda, Y.; Mukai, R.; Yoshihara, T.; Tobita, S. Ir(iii) complex-based oxygen imaging of living cells and ocular fundus with a gated ICCD camera. Photochem. Photobiol. Sci. 2018, 17, 846–853. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wu, K.; Pan, M.; Li, K.; Mo, J.T.; Duan, X.H.; He, H.Z.; Shen, J.; Su, C.Y. One-/two-photon excited cell membrane imaging and tracking by a photoactive nanocage. ACS Appl. Mater. Interfaces 2020, 12, 35873–35881. [Google Scholar] [CrossRef] [PubMed]
- Solomatina, A.I.; Kuznetsov, K.M.; Gurzhiy, V.V.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; Tunik, S.P. Luminescent organic dyes containing a phenanthro[9,10-D]imidazole core and [Ir(N^C)(N^N)]+ complexes based on the cyclometalating and diimine ligands of this type. Dalton Trans. 2020, 49, 6751–6763. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, I.S.; Chelushkin, P.S.; Sokolov, V.V.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; Tunik, S.P. Near-Infrared [Ir(N∧C)2(N∧N)]+ Emitters and Their Noncovalent Adducts with Human Serum Albumin: Synthesis and Photophysical and Computational Study. Organometallics 2019, 38, 3740–3751. [Google Scholar] [CrossRef]
- Hahn, U.; Luelf, H.; Winkler, H.D.F.; Schalley, C.A.; Vögtle, F.; De Cola, L. Encapsulation of luminescent homoleptic [Ru(dpp)3]2+-type chromophores within an amphiphilic dendritic environment. Chem. A Eur. J. 2012, 18, 15424–15432. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Mechanisms and dynamics of fluorescence quenching. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 331–351. ISBN 978-0-387-46312-4. [Google Scholar]
- Mogilner, A.; Manhart, A. Intracellular fluid mechanics: Coupling cytoplasmic flow with active cytoskeletal gel. Annu. Rev. Fluid Mech. 2018, 50, 347–370. [Google Scholar] [CrossRef] [Green Version]
- Ordóñez-Hernández, J.; Jiménez-Sánchez, A.; García-Ortega, H.; Sánchez-Puig, N.; Flores-Álamo, M.; Santillan, R.; Farfán, N. A series of dual-responsive Coumarin-Bodipy probes for local microviscosity monitoring. Dye. Pigment. 2018, 157, 305–313. [Google Scholar] [CrossRef]
- Kwapiszewska, K.; Szczepański, K.; Kalwarczyk, T.; Michalska, B.; Patalas-Krawczyk, P.; Szymański, J.; Andryszewski, T.; Iwan, M.; Duszyński, J.; Hołyst, R. Nanoscale viscosity of cytoplasm is conserved in human cell lines. J. Phys. Chem. Lett. 2020, 11, 6914–6920. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, W.L.; Chan, M.C.W. Luminescent oligo(ethylene glycol)-functionalized cyclometalated platinum(ii) complexes: Cellular characterization and mitochondria-specific localization. Chem. Commun. 2014, 50, 1711–1714. [Google Scholar] [CrossRef]
- Li, S.P.-Y.; Liu, H.-W.; Zhang, K.Y.; Lo, K.K.-W. Modification of luminescent iridium(iii) polypyridine complexes with discrete poly(ethylene glycol) (PEG) pendants: Synthesis, emissive behavior, intracellular uptake, and pegylation properties. Chem. A Eur. J. 2010, 16, 8329–8339. [Google Scholar] [CrossRef]
- Conway, J.R.W.; Warren, S.C.; Herrmann, D.; Murphy, K.J.; Cazet, A.S.; Vennin, C.; Shearer, R.F.; Killen, M.J.; Magenau, A.; Mélénec, P.; et al. Intravital imaging to monitor therapeutic response in moving hypoxic regions resistant to PI3K pathway targeting in pancreatic cancer. Cell Rep. 2018, 23, 3312–3326. [Google Scholar] [CrossRef]
- Kiessling, F.; Heilmann, M.; Lammers, T.; Ulbrich, K.; Subr, V.; Peschke, P.; Waengler, B.; Mier, W.; Schrenk, H.-H.; Bock, M.; et al. Synthesis and characterization of HE-24.8: A polymeric contrast agent for magnetic resonance angiography. Bioconjug. Chem. 2006, 17, 42–51. [Google Scholar] [CrossRef]
- Samudrala, R.; Zhang, X.; Wadkins, R.M.; Mattern, D.L. Synthesis of a non-cationic, water-soluble perylenetetracarboxylic diimide and its interactions with G-quadruplex-forming DNA. Bioorganic Med. Chem. 2007, 15, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Peverati, R.; Truhlar, D.G. Screened-exchange density functionals with broad accuracy for chemistry and solid-state physics. Phys. Chem. Chem. Phys. 2012, 14, 16187–16191. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]









| λabs (nm) | λexc (nm) a | λem (nm) b | τaer (μs) c | τdeg (μs) c | QYaer (%) b | QYdeg (%) b | kr, s−1 ×105 | knr, s−1 ×105 | |
|---|---|---|---|---|---|---|---|---|---|
| Ir1 | 252; 306sh; 344; 370; 441sh; 530; 570sh | 254; 344; 367; 442sh; 531; 550sh | 728; 790; 900sh | 2.34 | 3.23 | 8.4 | 12.2 | 0.38 | 2.72 |
| Ir2 | 253; 306sh; 345; 369; 441sh; 532; 570sh | 254; 344; 367; 442sh; 537; 555sh | 727; 789; 900sh | 2.16 | 3.28 | 8.2 | 11.9 | 0.36 | 2.73 |
| Ir2a | 253; 305sh; 345; 368; 440sh; 532; 570sh | 251; 343; 369; 441sh; 532; 556sh | 727; 789; 898sh | 2.13 | 3.24 | 8.1 | 11.7 | 0.36 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritchenkov, I.S.; Solomatina, A.I.; Kozina, D.O.; Porsev, V.V.; Sokolov, V.V.; Shirmanova, M.V.; Lukina, M.M.; Komarova, A.D.; Shcheslavskiy, V.I.; Belyaeva, T.N.; et al. Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging. Molecules 2021, 26, 2898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102898
Kritchenkov IS, Solomatina AI, Kozina DO, Porsev VV, Sokolov VV, Shirmanova MV, Lukina MM, Komarova AD, Shcheslavskiy VI, Belyaeva TN, et al. Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging. Molecules. 2021; 26(10):2898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102898
Chicago/Turabian StyleKritchenkov, Ilya S., Anastasia I. Solomatina, Daria O. Kozina, Vitaly V. Porsev, Victor V. Sokolov, Marina V. Shirmanova, Maria M. Lukina, Anastasia D. Komarova, Vladislav I. Shcheslavskiy, Tatiana N. Belyaeva, and et al. 2021. "Biocompatible Ir(III) Complexes as Oxygen Sensors for Phosphorescence Lifetime Imaging" Molecules 26, no. 10: 2898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26102898