Synthesis and Guest-Binding Properties of pH/Reduction Dual-Responsive Cyclophane Dimer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of pH/Reduction-Responsive Cyclophane Dimer
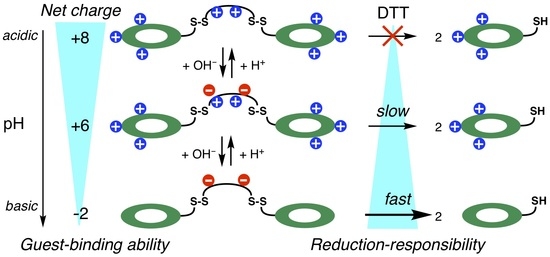
2.2. pH-Responsive Guest-Binding Behavior of 1
2.3. Thermodynamically Characterization of Guest-Binding Behavior of 1
2.4. Reduction-Responsive Guest-Binding Behavior of 1
3. Experimental Section
3.1. Materials
3.2. Cyclophane Derivative Tethered with Cystamine (4)
3.3. Cyclophane Dimer 1
3.4. Binding Constants of Cyclophane with TNS
3.5. General Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Lehn, J.-M.; Atwood, J.L.; Davis, J.E.D.; MacNicol, D.D.; Vögtle, F. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Gellman, S.H. Introduction: Molecular recognition. Chem. Rev. 1977, 97, 1231–1232. [Google Scholar] [CrossRef] [Green Version]
- Rebek, J., Jr. Host–guest chemistry of calixarene capsules. Chem. Commun. 2000, 637–643. [Google Scholar] [CrossRef]
- Biros, S.M.; Rebek, J., Jr. Structure and binding properties of water-soluble cavitands and capsules. Chem. Soc. Rev. 2007, 36, 93–104. [Google Scholar] [CrossRef]
- Huang, C.W.; Wu, P.W.; Su, W.H.; Zhu, C.Y.; Kuo, S.W. Stimuli-responsive supramolecular materials: Photo-tunable properties and molecular recognition behavior. Polym. Chem. 2016, 7, 795–806. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef]
- Hayashida, O.; Ogawa, N.; Uchiyama, M. Surface recognition and fluorescence sensing of histone by dansyl-appended cyclophane-based resorcinarene trimer. J. Am. Chem. Soc. 2007, 129, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Ariga, K.; Makita, T.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Review of advanced sensor devices employing nanoarchitectonics concepts. Beilstein J. Nanotechnol. 2019, 10, 2014–2030. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2017, 50, 7743–7765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Braegelman, A.S.; Webber, M.J. Spatially defined drug targeting by in situ host–guest chemistry in a living animal. ACS Cent. Sci. 2019, 5, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.L.; Xiao, X.; Cong, H.; Liang, L.L.; Cheng, K.; Cheng, X.J.; Ji, N.N.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Cucurbit[n]uril-based coordination chemistry: From simple coordination complexes to novel poly-dimensional coordination polymers. Chem. Soc. Rev. 2013, 42, 9480–9508. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Ni, X.L.; Xiao, X.; Huang, Y.; Zhu, Q.J.; Xue, S.F.; Tao, Z.; Lindoy, L.; Wei, G. Synthesis and separation of cucurbit[n]urils and their derivatives. Org. Biomol. Chem. 2016, 14, 4335–4364. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, A.; Shinkai, S. Novel cavity design using calix[n]arene skeletons: Toward molecular recognition and metal binding. Chem. Rev. 1997, 95, 1713–1734. [Google Scholar] [CrossRef]
- Sameni, S.; Jeunesse, C.; Matt, D.; Harrowfield, J. Calix[4]arene daisychains. Chem. Soc. Rev. 2009, 38, 2117–2146. [Google Scholar] [CrossRef]
- Perret, F.; Coleman, A.W. Biochemistry of anionic calix[n]arenes. Chem. Commun. 2011, 47, 7303–7319. [Google Scholar] [CrossRef]
- Cottet, K.; Macros, P.M.; Cragg, P.J. Fifty years of oxacalix[3]arenes: A review. Beilstein J. Org. Chem. 2012, 8, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Yamagishi, T.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Sathiyajith, C.W.; Shaikh, R.R.; Han, Q.; Zhang, Y.; Meguellati, K.; Yang, Y.W. Biological and related applications of pillar[n]arenes. Chem. Commun. 2017, 53, 677–696. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, B.; Wang, F.; Huang, F. Supramolecular polymers constructed from macrocycle-based host-guest molecular recognition motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef]
- Zhong, D.C.; Lu, T.B. Molecular recognition and activation by polyaza macrocyclic compounds based on host–guest interactions. Chem. Commun. 2016, 52, 10322–10337. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Smith, B.D. Molecular recognition using tetralactam macrocycles with parallel aromatic sidewalls. Beilstein J. Org. Chem. 2019, 15, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. [Google Scholar] [CrossRef]
- Shad, M.S.; Santhini, P.V.; Dehaen, W. 1,2,3-Triazolium macrocycles in supramolecular chemistry. Beilstein J. Org. Chem. 2019, 15, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mukhopadhyay, P.; Lin, S.; Isaacs, L. Reconfigurable four-component molecular switch based on pH-controlled guest swapping. Org. Lett. 2007, 9, 2349–2352. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.J.; Jiang, L.H. Stimuli-responsive supramolecular nanocapsules from amphiphilic calixarene assembly. J. Am. Chem. Soc. 2004, 126, 12724–12725. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Qi, L.; Zhong, W.; Lin, C.; Wang, R.; Wang, L. Stimuli-responsive nanocarriers constructed from pillar[n]arene-based supra-amphiphiles. Mater. Chem. Front. 2019, 3, 1973–1993. [Google Scholar] [CrossRef]
- Hayashida, O.; Hamachi, I. New Supramolecular Approach for Saccharide-directed Chemical Modification of Concanavalin A. Chem. Lett. 2003, 32, 632–633. [Google Scholar] [CrossRef]
- Hayashida, O.; Kaku, Y. Synthesis of Dabsyl-appended cyclophanes and their heterodimer formation with pyrene-appended cyclophanes. J. Org. Chem. 2013, 78, 10437–10442. [Google Scholar] [CrossRef]
- Hayashida, O.; Ichimura, K.; Sato, D.; Yasunaga, T. Synthesis, guest-binding, and reduction-responsive degradation properties of water-soluble cyclophanes having disulfide moieties. J. Org. Chem. 2013, 78, 5463–5469. [Google Scholar] [CrossRef]
- Hayashida, O.; Sato, D. Preparation and multivalently enhanced guest-binding affinity of water-soluble cyclophane heptadecamers. J. Org. Chem. 2008, 73, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, O. Bottom-Up Nanofabrication; Ariga, K., Nalwa, H.S., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2009; Volume 2, Chapter 18; pp. 423–436. [Google Scholar]
- Chinchio, M.; Czaplewski, C.; Liwo, A.; Oldziej, S.; Scheraga, H.A. Dynamic formation and breaking of disulfide bonds in molecular dynamics simulations with the UNRES force field. J. Chem. Theory Comput. 2007, 3, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Black, S.P.; Sanders, J.K.M.; Stefankiewicz, A.R. Disulfide exchange: Exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 2014, 43, 1861–1872. [Google Scholar] [CrossRef]
- Bracchi, M.E.; Fulton, D.A. Orthogonal breaking and forming of dynamic covalent imine and disulfide bonds in aqueous solution. Chem. Commun. 2015, 51, 11052–11055. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, O.; Ichimura, K. Synthesis and characterization of reduction-responsive cyclophane dimer based on disulfide linkage. Chem. Lett. 2012, 41, 1650–1651. [Google Scholar] [CrossRef]
- Hayashida, O.; Shibata, K. Stimuli-responsive supramolecular coaggregation and disaggregation of host–guest conjugates having a disulfide linkage. J. Org. Chem. 2020, 85, 5493–5502. [Google Scholar] [CrossRef]
- Odashima, K.; Itai, A.; Iitaka, Y.; Koga, K. Biomimetic studies using artificial systems. 3. Design, synthesis, and inclusion complex forming ability of a novel water-soluble paracyclophane possessing diphenylmethane skeletons. J. Org. Chem. 1991, 50, 4478–4484. [Google Scholar] [CrossRef]
- Tao, L.; Han, J.; Tao, F.M. Correlations and predictions of carboxylic acid pKa values using intermolecular structure and properties of hydrogen-bonded complexes. J. Phys. Chem. A 2008, 112, 775–782. [Google Scholar] [CrossRef]
- Hayashida, O.; Kojima, M.; Kusano, S. Biotinylated cyclophane: Synthesis, cyclophane-avidin conjugates, and their enhanced guest-binding affinity. J. Org. Chem. 2015, 80, 9722–9727. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]








| T/K | ||||
|---|---|---|---|---|
| pH | 288 | 298 | 308 | 318 |
| 3.8 | 1.6 × 105 | 9.6 × 104 | 4.9 × 104 | 2.4 × 104 |
| 7.4 | 1.0 × 105 | 6.0 × 104 | 4.0 × 104 | 2.3 × 104 |
| 10.7 | 3.3 × 104 | 2.4 × 104 | 1.7 × 104 | 1.2 × 104 |
| pH | ΔG, kJ mol−1 | ΔH, kJ mol−1 | TΔS, kJ mol−1 |
|---|---|---|---|
| 3.8 | −28.4 | −75.2 | −46.8 |
| 7.4 | −27.2 | −64.3 | −37.1 |
| 10.7 | −25.0 | −49.9 | −24.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashida, O.; Tanaka, Y.; Miyazaki, T. Synthesis and Guest-Binding Properties of pH/Reduction Dual-Responsive Cyclophane Dimer. Molecules 2021, 26, 3097. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113097
Hayashida O, Tanaka Y, Miyazaki T. Synthesis and Guest-Binding Properties of pH/Reduction Dual-Responsive Cyclophane Dimer. Molecules. 2021; 26(11):3097. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113097
Chicago/Turabian StyleHayashida, Osamu, Yudai Tanaka, and Takaaki Miyazaki. 2021. "Synthesis and Guest-Binding Properties of pH/Reduction Dual-Responsive Cyclophane Dimer" Molecules 26, no. 11: 3097. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26113097