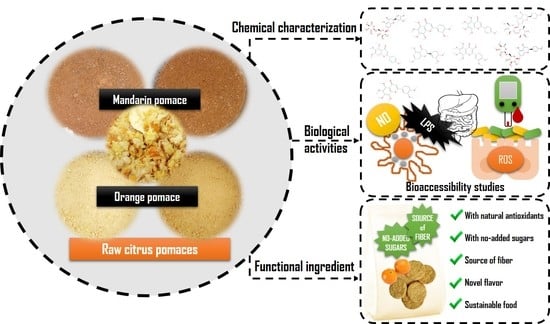
In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Citrus Pomaces by UHPLC-MS/MS Analysis
2.2. Bioaccessibility of Antioxidants from Citrus Pomaces
2.3. Bioaccessibility of Anti-Inflammatory Compounds Composing Citrus Pomaces
2.4. Bioaccessibility of Inhibitors of the Enzymes α-Glucosidase and α-Amylase Composing Citrus Pomaces
2.5. Bioaccessibility of Bioactive Compounds Composing Biscuits Containing Orange Pomace as a Food Ingridient
2.6. Food Sensory Quality
3. Materials and Methods
3.1. Materials
3.2. Samples
3.3. Methods
3.3.1. Analysis of Individual Phenolic Compounds Composing Citrus Pomaces
3.3.2. Analysis of Bioaccessibility of Health Promoting Compounds
Antioxidant Compounds
Anti-Inflammatory Compounds
Inhibitors of Carbohydrases Enzymatic Activity
3.3.3. Assessment of Food Sensory Quality
3.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fernández-Fernández, A.M.; Dellacassa, E.; Medrano-Fernandez, A.; Del Castillo, M.D. Citrus Waste Recovery for Sustainable Nutrition and Health. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 193–211. ISBN 9781119534105. [Google Scholar]
- WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 25 November 2019).
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-kB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Gulsunoglu, Z.; Karbancioglu-Guler, F.; Raes, K.; Kilic-Akyilmaz, M. Soluble and insoluble-bound phenolics and antioxidant activity of various industrial plant wastes. Int. J. Food Prop. 2019, 22, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Yeo, J.D. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds–A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Ye, X.; Cao, D.; Zhao, X.; Song, F.; Huang, Q.; Fan, G.; Wu, F. Chemical fingerprint and metabolic profile analysis of Citrus reticulate “Chachi” decoction by HPLC-PDA-IT-MS n and HPLC-Quadrupole-Orbitrap-MS method. J. Chromatogr. B 2014, 970, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.J.; Pan, Y.; Zhou, Z. Identification of the chemical compositions of Ponkan peel by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Anal. Methods 2016, 8, 893–903. [Google Scholar] [CrossRef]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and delivery of mandarin (Citrus reticulata Blanco) peel extracts by encapsulation of whey protein concentrate nanoparticles. LWT Food Sci. Technol. 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-T.; Chu, H.-L.; Chyau, C.-C.; Chu, C.-C.; Duh, P.-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, W.; Huang, H.; Ye, X.; Sun, P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Viuda-Martos, M.; Angel Pérez-Alvarez, J.; Fernández-López, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Y.; Niu, X.; Tang, H.; Meydani, S.N.; Wu, D. Dietary naringenin supplementation attenuates experimental autoimmune encephalomyelitis by modulating autoimmune inflammatory responses in mice. J. Nutr. Biochem. 2018, 54, 130–139. [Google Scholar] [CrossRef]
- Gosslau, A.; Chen, K.Y.; Ho, C.-T.; Li, S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci. Hum. Wellness 2014, 3, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, V.M.; Moala, T.; e Paiva Caria, C.R.; Moura, C.S.; Amaya-Farfan, J.; Gambero, A.; Macedo, G.A.; Macedo, J.A. Biotransformed citrus extract as a source of anti-inflammatory polyphenols: Effects in macrophages and adipocytes. Food Res. Int. 2017, 97, 37–44. [Google Scholar] [CrossRef]
- Ho, S.-C.; Kuo, C.-T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Mokdad Bzéouich, I.; Mustapha, N.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory potential of hesperetin and chrysin through the cellular and humoral response. Eur. J. Pharmacol. 2017, 812, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Abraham, A. Inhibition of LPS induced pro-inflammatory responses in RAW 264.7 macrophage cells by PVP-coated naringenin nanoparticle via down regulation of NF-κB/P38MAPK mediated stress signaling. Pharmacol. Rep. 2017, 69, 908–915. [Google Scholar] [CrossRef]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus flavonoids collectively dominate the α-amylase and α-glucosidase inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Leporini, M.; Sicari, V.; Falco, T.; Pellicanò, T.M.; Tundis, R. Investigating the in vitro hypoglycaemic and antioxidant properties of Citrus × clementina Hort. juice. Eur. Food Res. Technol. 2018, 244, 523–534. [Google Scholar] [CrossRef]
- Nagarajaiah, S.B.; Prakash, J. Chemical Composition and Bioactivity of Pomace from Selected Fruits. Int. J. Fruit Sci. 2016, 16, 423–443. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Liu, B.; Jones, P.; Persaud, S.J.; Mastellone, V.; Lombardi, P.; Houghton, P.J.; et al. medica cv Diamante peel chemical composition and influence on glucose homeostasis and metabolic parameters. Food Chem. 2011, 124, 1083–1089. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Hochkogler, C.M.; Somoza, V.; del Castillo, M.D. Biscuits with no added sugar containing stevia, coffee fibre and fructooligosaccharides modifies α-glucosidase activity and the release of GLP-1 from HuTu-80 cells and serotonin from Caco-2 cells after in vitro digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.M.; Leelavathi, K.; Prasada Rao, U.J.S. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; de Oliveira Rios, A.; Silveira Thys, R.C.; Hickmann Flôres, S. Effects of orange by-product fiber incorporation on the functional and technological properties of pasta. Food Sci. Technol. 2015, 35, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-targeted glycosidic profiling of international wines using neutral loss-high resolution mass spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of antioxidant, antidiabetic, antiobesity, and anti-inflammatory properties of a Tannat winemaking by-product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]








| Compounds 1 | Clemenule Mandarin 2 | Ortanique Mandarin 2 | Navel Orange 2 | Valencia Orange 2 | RT | λmax (nm) (UHPLC-DAD) | [M + H]+ (m/z) | Fragments (m/z) |
|---|---|---|---|---|---|---|---|---|
| nariturin-4-glucoside/naringin glucoside | 0.000078 | 0.000391 | 0.000755 | 0.000528 | 9.6 | 266sh, 359 | 741.2248 | 271.0639, 151.0035 |
| Rutin | 0.003400 | 0.005722 | 0.001625 | 0.001366 | 10.3 | 285, 325 | 609.1461 | 301.0350, 271.0257 |
| Eriocitrin/ Neoeriocitrin 1 | 0.001715 | 0.000608 | 0.001164 | 0.000806 | 10.6 | 266, 336/268, 334 | 595.1668 | 287.0580, 151.0034 |
| Rhoifolin/Isorhoifolin | 0.000855 | 0.000944 | 0.000236 | 0.000086 | 11.0 | 284, 330/284 | 579.1708 | 271,0595 |
| Naringin/Narirutin | 0.008453 | 0.029204 | 0.030457 | 0.021736 | 11.2 | 250, 268, 342 | 579.1719 | 271.0637, 151.0035 |
| Diosmin isomer 1 | 0.000783 | 0.001677 | 0.000839 | 0.000797 | 11.3 | 285, 340/284 | 607.1668 | 299.0580, 284.0338 |
| Eriocitrin/ Neoeriocitrin 2 | 0.000187 | 0.000048 | 0.000060 | 0.000056 | 11.3 | 268, 342 | 595.1668 | 287.0580, 151.0034 |
| Diosmin isomer 2 | 0.000889 | 0.000327 | 0.000441 | 0.000393 | 11.4 | 283, 326/283, 332 | 607.1668 | 299.0580, 284.0338 |
| Hesperidin/ Neohesperidin | 0.059179 | 0.046458 | 0.071641 | 0.064532 | 11.6 | 283, 328/266sh, 354 | 609.1825 | 301.0739, 151.0035 |
| Poncirin/ Isosakuranetin-7-O-rutinoside | 0.000270 | 0.002624 | 0.002497 | 0.001776 | 13.1 | 270sh, 338/214, 328/285, 331 | 593.1876 | 285.0763 |
| Isosinensetin/ Sinensetin/ Tangeretin 1 | 0.015972 | 0.015961 | 0.010084 | 0.010829 | 16.2 | 270sh, 338/214, 328/285, 331 | 373.1282 | 343.0806, 153.0181 |
| Isosinensetin/ Sinensetin/ Tangeretin 2 | n.d. | 0.048355 | 0.058543 | 0.065486 | 16.7 | 272, 324 | 373.1282 | 343.0806, 153.0181 |
| Nobiletin | 0.081665 | 0.099307 | 0.073267 | 0.079792 | 17.3 | 268sh, 342 | 403.1387 | 373.091, 183.0288 |
| Heptamethoxyflavone | 0.072137 | 0.010010 | 0.019473 | 0.018539 | 17.6 | 272, 302 | 433.1493 | 403.1019, 418.1251 |
| Tetramethylscutellarein | 0.033105 | 0.085489 | 0.049083 | 0.047840 | 17.7 | 270sh, 338/214, 328/285, 331 | 343.1176 | 313.0701, 153.0180 |
| Isosinensetin/ Sinensetin/ Tangeretin 3 | 0.035461 | 0.072075 | 0.024060 | 0.024608 | 18.1 | 266sh, 359 | 373.1282 | 343.0806, 153.0181 |
| TIC | 823166557 | 884024903 | 878913863 | 817363291 |
| Samples | α-Glucosidase (IC50, mg/mL) | α-Amylase (IC50, mg/mL) | ||
|---|---|---|---|---|
| Undigested | Digested | Undigested | Digested | |
| Clemenule mandarin | 4.92 ± 0.27 b | 3.97 ± 0.97 a | 70.19 ± 11.16 bc | 58.04 ± 2.09 a |
| Ortanique mandarin | 3.42 ± 0.64 a | 4.93 ± 0.41 a | 50.07 ± 2.42 ab | 105.68 ± 16.03 b |
| Navel orange | 10.84 ± 1.19 c | 11.42 ± 0.89 b | 5.19 ± 0.22 a | 62.00 ± 1.62 a |
| Valencia orange | 5.19 ± 1.98 b | 5.09 ± 0.39 a | 77.57 ± 15.27 c | 101.17 ± 4.70 b |
| Ingredients | Control | Navel | Valencia |
|---|---|---|---|
| g/100 g | |||
| Butter | 10 | 10 | 10 |
| Sunflower oil | 4.25 | 4.25 | 4.25 |
| Egg | 14 | 14 | 14 |
| Baking powder | 0.5 | 0.5 | 0.5 |
| Salt | 0.08 | 0.08 | 0.08 |
| Sweetener | 4 | 4 | 4 |
| Wheat flour | 62.17 | 52.17 | 52.17 |
| By-product | 0 | 10 | 10 |
| Inulin | 5 | 5 | 5 |
| Total | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; del Castillo, M.D. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules 2021, 26, 3480. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123480
Fernández-Fernández AM, Dellacassa E, Nardin T, Larcher R, Gámbaro A, Medrano-Fernandez A, del Castillo MD. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules. 2021; 26(12):3480. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123480
Chicago/Turabian StyleFernández-Fernández, Adriana Maite, Eduardo Dellacassa, Tiziana Nardin, Roberto Larcher, Adriana Gámbaro, Alejandra Medrano-Fernandez, and María Dolores del Castillo. 2021. "In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits" Molecules 26, no. 12: 3480. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123480