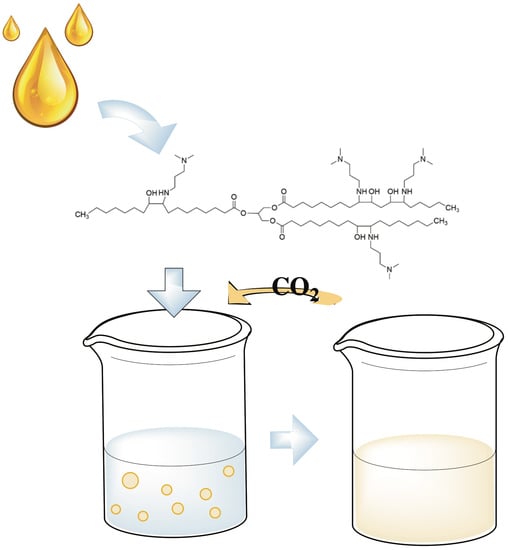
Soybean-Oil-Based CO2-Switchable Surfactants with Multiple Heads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Structure and CO2-Switching
2.2. Surface Activity
2.3. Viscosity-Enhancing Ability
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dib, N.; Falcone, R.D.; Acuña, A.; García-Río, L. The ionic liquid-surfactant bmim-AOT and nontoxic lipophilic solvents as components of reverse micelles alternative to the traditional systems. A study by 1H NMR spectroscopy. J. Mol. Liq. 2020, 304, 112762. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W. Study on osmotic pressure of non-ionic and ionic surfactant solutions in the micellar and microemulsion regions. Fluid Phase Equilib. 2008, 263, 231–235. [Google Scholar] [CrossRef]
- Goloub, T.P.; Pugh, R.J. The role of the surfactant head group in the emulsification process: Binary (nonionic–ionic) surfactant mixtures. J. Colloid Interface Sci. 2005, 291, 256–262. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Gao, W. Experimental investigation on transport property and emulsification mechanism of polymeric surfactants in porous media. J. Petrol. Sci. Eng. 2020, 186, 106687. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ling, Y.T.Q.; Heng, Y.X. An environment-friendly palm fatty acid-based polymeric surfactants for coating applications: Physicochemical, surface tension and low-foaming properties. J. Polym. Environ. 2019, 27, 2707–2719. [Google Scholar] [CrossRef]
- Lyu, B.; Liu, H.; Li, P.; Gao, D.; Ma, J. Preparation and properties of polymeric surfactants: A potential corrosion inhibitor of carbon steel in acidic medium. J. Ind. Eng. Chem. 2019, 80, 411–424. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Y.; Wang, W.; Zhu, L.; Liu, H.; Yang, S. Fluorinated polyhedral oligomeric silsesquioxanes end-capped poly(ethylene oxide giant surfactants: Precise synthesis and interfacial behaviors. Polymer 2020, 186, 122055. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Zhu, L.; Fan, Y.; Wang, Y. Partition and solubilization of phospholipid vesicles by noncovalently constructed oligomeric-like surfactants. Langmuir 2020, 36, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Ribosa, I.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of new cleavable betainate cationic oligomeric surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, W.; Yang, H.; Huang, Z.; Zhou, B.; Bauyrzhan, S. Emulsification and stabilization mechanism of crude oil emulsion by surfactant synergistic amphiphilic polymer system. Colloids Surf. A 2021, 609, 125726. [Google Scholar] [CrossRef]
- Kumae, D.; Abdul Rub, M. Influence of dimeric gemini surfactant micelles on the study of nickel-glycylleucine dipeptide and ninhydrin. J. Dispersion Sci. Technol. 2020, 41, 1559–1567. [Google Scholar]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Wang, D.; Feng, L.; Song, B.; Pei, X.; Cui, Z.; Xie, D. Viscoelastic lyotropic liquid crystals formed in a bio-based trimeric surfactant system. Soft Matter. 2019, 15, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, D.; Pei, X.; Song, B.; Cui, Z. Self-assembly and rheological behavior of oleic acid-based pseudo-tetrameric surfactants. Colloid Polym. Sci. 2018, 297, 125–132. [Google Scholar] [CrossRef]
- Wattebled, L.; Laschewsky, A.; Moussa, A.; Habib-Jiwan, J.L. Aggregation numbers of cationic oligomeric surfactants: A time-resolved fluorescence quenching study. Langmuir 2006, 22, 2551–2557. [Google Scholar] [CrossRef]
- Lamal, M.S.; Hussain, S.S.; Fogang, L.T.; Sultan, A.S. Impact of spacer and hydrophobic tail on interfacial and rheological properties of cationic amido-amine gemini surfactants for EOR application. Tenside Surfact. Det. 2018, 55, 491–497. [Google Scholar]
- Iglauer, S.; WU, Y.; Shuler, P.; Tang, Y.; Goddard III, W.A. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J. Petrol. Sci. Eng. 2009, 71, 23–29. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Gong, H.; Wu, D.; Wang, Y. Production of ultra-low interfacial tension between crude oil and mixed brine solution of Triton X–100 and its oligomer Tyloxapol with cetyltrimethylammonium bromide induced by hydrolyzed polyacrylamide. Colloids Surf. A 2008, 332, 90–97. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Zakharova, L.Y.; Khairutdinova, E.I.; Lukashenko, S.S.; Sinyashin, O.G. Supramolecular systems based on gemini surfactants for enhancing solubility of spectral probes and drugs in aqueous solution. Colloids Surf. A 2016, 510, 33–42. [Google Scholar] [CrossRef]
- Guo, S.; Sun, X.; Zou, Q.; Zhang, J.; Ni, H. Antibacterial activities of five cationic gemini surfactants with ethylene glycol bisacetyl spacers. J. Surfactants Deterg. 2014, 17, 1089–1097. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, D.; Ralston, J.; Liu, Q.; Xu, Z. CO2-responsive surfactants with tunable switching pH. J. Colloid Interface Sci. 2019, 557, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Grishkewich, N.; Tam, K.C.; Yuan, J.; Mao, Z.; Sui, X. CO2-responsive cellulose nanofibers aerogels for switchable oil–water separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, G.; Yang, J.; Xu, Z.; Sun, D. CO2-responsive aqueous foams stabilized by pseudogemini surfactants. J. Colloid Interface Sci. 2018, 536, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Tian, Q.; Feng, Y.; Yin, H.; Lu, G. CO2-switchable foams stabilized by a long-chain viscoelastic surfactant. J. Colloid Interface Sci. 2018, 523, 65–74. [Google Scholar] [CrossRef]
- Calculated Using ACD/I−Lab. Available online: https://ilab.acdlabs.com (accessed on 17 June 2021).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Huang, X.; Quan, H.; Su, X. Soybean-Oil-Based CO2-Switchable Surfactants with Multiple Heads. Molecules 2021, 26, 4342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144342
Huang H, Huang X, Quan H, Su X. Soybean-Oil-Based CO2-Switchable Surfactants with Multiple Heads. Molecules. 2021; 26(14):4342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144342
Chicago/Turabian StyleHuang, Huiyu, Xiaoling Huang, Hongping Quan, and Xin Su. 2021. "Soybean-Oil-Based CO2-Switchable Surfactants with Multiple Heads" Molecules 26, no. 14: 4342. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26144342