Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon
Abstract
:1. Introduction
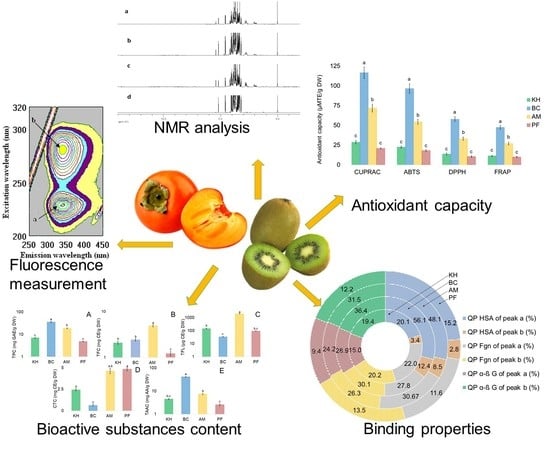
2. Results and Discussion
2.1. Identification of Bioactive Compounds in Fruit Extracts
2.2. Principal Component Analysis (PCA) and Multivariate Data Analysis (MVDA)
2.3. Determination of Bioactive Compounds
2.4. Antioxidant Capacities of Investigated Samples
2.5. Quenching Properties of Phenolic Compounds of Investigated Fruits with Human Serum Proteins
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sampling and NMR Metabolomics
3.3. Determination of Bioactive Compounds
3.4. Determination of Antioxidant Capacities
3.5. Fluorometric Studies
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nardozza, S.; Cooney, J.; Boldingh, H.L.; Hewitt, K.G.; Trower, T.; Jones, D.; Thrimawithana, A.H.; Allan, A.C.; Richardson, A.C. Phytohormone and transcriptomic analysis reveals endogenous cytokinins affect kiwifruit growth under restricted carbon supply. Metabolites 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, V.; Gorji, S.G.; Daygon, V.D.; Fitzgerald, M. Untargeted and targeted metabolomic profiling of australian indigenous fruits. Metabolites 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Vázquez-Manjarrez, N.; Vázquez-Manjarrez, N.; Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Garcia-Aloy, M.; Mattivi, F.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; et al. Biomarkers of intake for tropical fruits. Genes Nutr. 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Jesion, I.; Gorinstein, S. Nutraceutical value of persimmon (Diospyros kaki Thunb.) and its influence on some indices of atherosclerosis in an experiment on rats fed cholesterol-containing diet. Adv. Hortic. Sci. 2008, 22, 250–254. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, E.K.; Lim, Y.; Shin, J.Y.; Jung, H.A.; Park, S.Y.; Lee, J.H.; Choe, J.S.; Kwon, O. Metabolites profiling and hypolipidemic/hypocholesterolemic effects of persimmon (Diosyros kaki Thumb.) by different processing procedures: In vitro and in vivo studies. J. Nutr. Health 2018, 51, 275–286. [Google Scholar] [CrossRef]
- Yoshimura, M.; Mochizuki, A.; Amakura, Y. Identification of phenolic constituents and inhibitory activity of persimmon calyx and Shiteito against tumor cell proliferation. Chem. Pharm. Bull. 2021, 32–39. [Google Scholar] [CrossRef]
- Hunter, D.C.; Greenwood, J.; Zhang, J.; Skinner, M.A. Antioxidant and “natural protective” properties of kiwifruit. Curr. Top. Med. Chem. 2011, 11, 1811–1820. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Koda, M.; Furihata, K.; Wei, F.; Miyakawa, T.; Tanokura, M. Metabolic discrimination of mango juice from various cultivars by band-selective NMR spectroscopy. J. Agric. Food Chem. 2012, 60, 1158–1166. [Google Scholar] [CrossRef]
- Ryu, S.; Furihata, K.; Koda, M.; Wei, F.; Miyakawa, T.; Tanokura, M. NMR-based analysis of the chemical composition of Japanese persimmon aqueous extracts. Magn. Reson. Chem. 2016, 54, 213–221. [Google Scholar] [CrossRef]
- Ryu, S.; Muramatsu, T.; Furihata, K.; Wei, F.; Koda, M.; Miyakawa, T.; Tanokura, M. NMR-based metabolic profiling and comparison of Japanese persimmon cultivars. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Abdul Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S.; Namieśnik, J.; et al. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, P.; Du, K.; Li, M.; Xie, Y.; Gao, P. Nutritional component analyses of kiwifruit in different development stages by metabolomic and transcriptomic approaches. J. Sci. Food Agric. 2020, 100, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Rimac, H.; Debeljak, Ž.; Šakić, D.; Weitner, T.; Gabričević, M.; Vrček, V.; Zorc, B.; Bojić, M. Structural and electronic determinants of flavonoid binding to human serum albumin: An extensive ligand-based study. RSC Adv. 2016, 6, 75014–75022. [Google Scholar] [CrossRef] [Green Version]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Shahidi, F.; Ramakrishnam, V.V.; Oh, W.Y. Bioavailability and Metabolism of Food Bioactives and their Health Effects: A Review. J. Food Bioact. 2019, 8, 6–41. [Google Scholar] [CrossRef] [Green Version]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yang, F.; Liu, C.; Xu, X.; Yamamoto, K. Molecular structure-affinity relationship of natural polyphenols for bovine γ-globulin. Mol. Nutr. Food Res. 2011, 55, S86–S92. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, Y.; Wang, H.; Yuan, Y.; Yang, F.; Zhang, C.; Yamamoto, K. Noncovalent interaction of dietary polyphenols with common human plasma proteins. J. Agric. Food Chem. 2011, 59, 10747–10754. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Caspi, A.; Libman, I.; Lerner, H.T.; Huang, D.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Feng, S.; et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: Studies in vitro and in humans. J. Agric. Food Chem. 2006, 54, 1887–1892. [Google Scholar] [CrossRef]
- Shiyovich, A.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Kornowski, R.; Perl, L. Changes over time in serum albumin levels predict outcomes following percutaneous coronary intervention. J. Cardiol. 2020, 75, 381–386. [Google Scholar] [CrossRef]
- Deveci, B.; Gazi, E. Relation between globulin, fibrinogen, and albumin with the presence and severity of coronary artery disease. Angiology 2021, 72, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fan, C.-M.; Guo, H.; Fan, W.-N.; Li, M.-L.; Cui, G.-X. Fibrinogen-to-albumin ratio predicts long-term outcomes for patients with ST-elevation myocardial infarction and multivessel disease: A prospective observational cohort study. Exp. Ther. Med. 2021, 21, 465. [Google Scholar] [CrossRef]
- Gorinstein, S.; Haruenkit, R.; Poovarodom, S.; Park, Y.S.; Vearasilp, S.; Suhaj, M.; Ham, K.S.; Heo, B.G.; Cho, J.Y.; Jang, H.G. The comparative characteristics of snake and kiwi fruits. Food Chem. Toxicol. 2009, 47, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Puigdomenech, E.; Notario-Pacheco, B.; Mendizabal-Gallastegui, N.; De La Cal De La Fuente, A.; Otegui-Ilarduya, L.; Maderuelo-Fernandez, J.A.; De Cabo Laso, A.; et al. Effects of kiwi consumption on plasma lipids, fibrinogen and insulin resistance in the context of a normal diet. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L. Tannic Acid Inhibits Protein Disulfide Isomerase, Platelet Activation and Thrombus Formation. Blood 2018, 132, 2418. [Google Scholar] [CrossRef]
- Luo, X.; Du, C.; Cheng, H.; Chen, J.; Lin, C. Study on the anticoagulant or procoagulant activities of type II phenolic acid derivatives. Molecules 2017, 22, 2047. [Google Scholar] [CrossRef] [Green Version]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, F.R.; Delfini, M. Metabolic profiling and outer pericarp water state in zespri, CI.GI, and hayward kiwifruits. J. Agric. Food Chem. 2013, 61, 1727–1740. [Google Scholar] [CrossRef]
- Maulidiani, M.; Mediani, A.; Abas, F.; Park, Y.K.Y.S.; Park, Y.K.Y.S.; Kim, Y.M.; Gorinstein, S. 1H-NMR and antioxidant profiles of polar and non-polar extracts of persimmon (Diospyros kaki L.)—Metabolomics study based on cultivars and origins. Talanta 2018, 184, 277–286. [Google Scholar] [CrossRef]
- Park, Y.K.Y.S.; Leontowicz, M.; Leontowicz, H.; Ham, K.S.; Kang, S.G.; Park, Y.K.Y.S.; Rombolà, A.D.; Katrich, E.; Gorinstein, S. Fluorescence and ultraviolet spectroscopic evaluation of phenolic compounds, antioxidant and binding activities in some kiwi fruit cultivars. Spectrosc. Lett. 2015, 48, 586–592. [Google Scholar] [CrossRef]
- Li, D.; Zhu, F. Physicochemical, functional and nutritional properties of kiwifruit flour. Food Hydrocoll. 2019, 92, 250–258. [Google Scholar] [CrossRef]
- Hettihewa, S.K.; Hemar, Y.; Vasantha Rupasinghe, H.P. Flavonoid-rich extract of actinidia macrosperma (a wild kiwifruit) inhibits angiotensin-converting enzyme in vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.Y.S.; Ham, K.S.; Park, Y.K.Y.S.; Leontowicz, H.; Leontowicz, M.; Namieśnik, J.; Katrich, E.; Gorinstein, S. The effects of treatment on quality parameters of smoothie-type “Hayward” kiwi fruit beverages. Food Control 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Pinto, D.; Sut, S.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Actinidia arguta Pulp: Phytochemical Composition, Radical Scavenging Activity, and in Vitro Cells Effects. Chem. Biodivers. 2021, 18, e2000925. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen kiwifruit (actinidia) cultivars—comparative analysis of fifteen kiwifruit (actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia arguta)—a Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Lan, T.; Ju, Y.; Cheng, G.; Que, Z.; Geng, T.; Fang, Y.; Sun, X. Comparison of the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms of products: Towards making kiwifruit more nutritious and functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yuan, Q.; Yang, Y.L.; Han, Q.H.; He, J.L.; Zhao, L.; Zhang, Q.; Liu, S.X.; Lin, D.R.; Wu, D.T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef] [Green Version]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Chen, L.; He, Y.; Li, X.; Lv, Z.; Yi, S.; Zhong, M.; Huang, C.; Jia, D.; Qu, X.; et al. Three metabolic pathways are responsible for the accumulation and maintenance of high AsA content in kiwifruit (Actinidia eriantha). BMC Genom. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Iwasawa, H.; Morita, E.; Yui, S.; Yamazaki, M. Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol. Pharm. Bull. 2011, 34, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa “Hayward” and Actinidia eriantha “Bidan”. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K. Secondary Metabolite Components of Kiwifruit. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 68, pp. 101–124. [Google Scholar]
- Gorinstein, S.; Kulasek, G.W.; Bartnikowska, E.; Leontowicz, M.; Zemser, M.; Morawiec, M.; Trakhtenberg, S. The effects of diets, supplemented with either whole persimmon or phenol-free persimmon, on rats fed cholesterol. Food Chem. 2000, 70, 303–308. [Google Scholar] [CrossRef]
- Persic, M.; Jakopic, J.; Hudina, M. The effect of post-harvest technologies on selected metabolites in persimmon (Diospyros kaki Thunb.) fruit. J. Sci. Food Agric. 2019, 99, 854–860. [Google Scholar] [CrossRef]
- Esteban-Muñoz, A.; Sánchez-Hernández, S.; Samaniego-Sánchez, C.; Giménez-Martínez, R.; Olalla-Herrera, M. Differences in the phenolic profile by uplc coupled to high resolution mass spectrometry and antioxidant capacity of two diospyros kaki varieties. Antioxidants 2021, 10, 31. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Delgado-Licon, E.; Leticia Martinez Ayala, A.; Tapia, M.S.; Martín-Belloso, O.; Trakhtenberg, S.; Gorinstein, S. Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. LWT—Food Sci. Technol. 2006, 39, 748–755. [Google Scholar] [CrossRef]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Park, Y.S.; Kim, Y.M.; Paśko, P.; Deutsch, J.; Katrich, E.; Gorinstein, S. Unraveling the Antioxidant, Binding and Health-Protecting Properties of Phenolic Compounds of Beers with Main Human Serum Proteins: In Vitro and In Silico Approaches. Molecules 2020, 25, 4962. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.J.; Hwang, I.G.; Song, J.; Cho, S.M. Cholesterol-lowering effect of astringent persimmon fruits (Diospyros kaki Thunb.) extracts. Food Sci. Biotechnol. 2017, 26, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R.; Shin, S.H.; Roh, S.S. Diospyros kaki and Citrus unshiu Mixture Improves Disorders of Lipid Metabolism in Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2020, 2020, 8812634. [Google Scholar] [CrossRef]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Viuda-Martos, M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb. vars. ‘Rojo Brillante’ and ‘Triumph’ co-products. J. Sci. Food Agric. 2018, 98, 504–510. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Zhai, X.; Pang, Y.; Yang, X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr. Res. 2019, 63, 1577. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leontowicz, M.; Leontowicz, H.; Jesion, I.; Bielecki, W.; Najman, K.; Latocha, P.; Park, Y.S.; Gorinstein, S. Actinidia arguta supplementation protects aorta and liver in rats with induced hypercholesterolemia. Nutr. Res. 2016, 36, 1231–1242. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Chung, K.H.; Nam, E.Y.; Kwon, J.H.; Hur, Y.Y.; Kwon, S., Il; Kim, Y.K.; Ma, K.B.; Yun, S.H.; Lee, M.H.; Park, Y.S.; et al. The History, Current Status and Future Prospects of Fruit Breeding in Korea. Korean J. Breed. Sci. 2020, 52, 144–160. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, Y.K.Y.S.; Park, Y.K.Y.S.; Ham, K.S.; Kang, S.G.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Phytochemical analysis of two main varieties of persimmon and kiwifruit and their antioxidative and quenching capacities. Eur. Food Res. Technol. 2020, 246, 1259–1268. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, Y.K.Y.S.; Park, Y.K.Y.S.; Ham, K.S.; Kang, S.G.; Shafreen, R.M.B.; Lakshmi, S.A.; Gorinstein, S. Characterization of bioactive ligands with antioxidant properties of kiwifruit and persimmon cultivars using in vitro and in silico studies. Appl. Sci. 2020, 10, 4218. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feucht, W.; Polster, J. Nuclei of Plants as a Sink for Flavanols. Z. Naturforsch. Sect. C J. Biosci. 2001, 56, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoǧlu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids-La(III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maulidiani, H.; Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of three pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2012, 60, 410–417. [Google Scholar] [CrossRef]










| No. | Compound | CAS | Structure | δH(ppm), Multiplicity, J Value (Hz) |
|---|---|---|---|---|
| 1 | Phenylalanine | 63-91-2 |  | 7.40, m (2H) 7.35, m 7.30, d, 7.4 (2H) |
| 2 | Kaempferol | 520-18-3 |  | 8.01, d, 8.0 6.95, d, 8.0 6.32, br d (small d) 6.10, br d (small d) |
| 3 | Rutin | 153-18-4 |  | 7.65, d, 2.0 7.60, dd, 6.82, d, 8.5 6.38, d, 6.19, d, 1.05, d, 7.0 4.51, br s (small d) 5.05, d, 8.0 |
| 4 | Tryptophan | 73-22-3 |  | 7.70, d, 8.0 7.54, d, 8.0 7.20, t, 7.0 |
| 5 | Tyrosine | 60-18-4 |  | 3.94, m 7.15, d, 8.0 6.82, d, 8.0 |
| 6 | Caffeic acid derivatives |  | 7.57, d, 13.0 7.28, br s (small d) 7.22, d, 8.0 6.95, d, 8.0 6.55, d, 13.0 | |
| 7 | Protocatechuic acid | 99-50-3 |  | 7.39, br s (small d) 7.35, br d (dd), 8.0 6.92, d, 8.0 |
| 8 | Catechol | 120-80-9 |  | 6.77–6.84, m 4.52, d, 7.20 2.94, dd, 15.7, 6.2 2.47, dd, 15.0, 8.0 |
| 9 | Syringic acid | 530-57-4 |  | 7.26, s, 2H 3.89, s |
| 10 | Afzelechin | 2545-00-8 |  | 2.83; 2.80; 2.79, dd,15.6, 4.8 2.68, dd 6.85, d, 8.0 (2H) 7.17, d, 8.0 (2H) |
| 11 | Kaempferol derivatives |  | 6.97, d, 2.7 6.46, d, 2.7 | |
| 12 | Quercetin derivatives |  | 7.52, d, 3.5 6.66, d, 3.5 | |
| 13 | Gallic acid | 149-91-7 |  | 7.01 (s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Abas, F.; Park, Y.-S.; Park, Y.-K.; Ham, K.-S.; Kang, S.-G.; Lubinska-Szczygeł, M.; Ezra, A.; Gorinstein, S. Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon. Molecules 2021, 26, 4405. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154405
Kim Y-M, Abas F, Park Y-S, Park Y-K, Ham K-S, Kang S-G, Lubinska-Szczygeł M, Ezra A, Gorinstein S. Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon. Molecules. 2021; 26(15):4405. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154405
Chicago/Turabian StyleKim, Young-Mo, Faridah Abas, Yong-Seo Park, Yang-Kyun Park, Kyung-Sik Ham, Seong-Gook Kang, Martyna Lubinska-Szczygeł, Aviva Ezra, and Shela Gorinstein. 2021. "Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon" Molecules 26, no. 15: 4405. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154405