Enhancement of Activity and Development of Low Pt Content Electrocatalysts for Oxygen Reduction Reaction in Acid Media †
Abstract
:1. Introduction
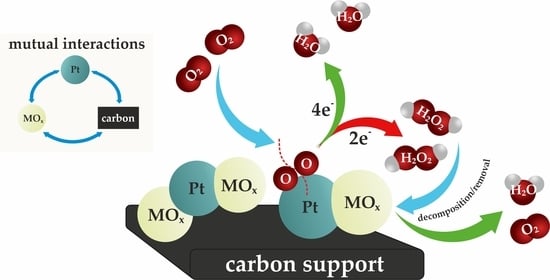
2. Pt-Metal Oxides (Pt-MO) Hybrid Catalysts
3. Alloyed Pt Nanostructures
4. Heteroatom Doped Carbon Carriers
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Epping Martin, K.; Kopasz, J.P.; McMurphy, K.W. Status of Fuel Cells and the Challenges Facing Fuel Cell Technology Today making further progress in eliminating cost, durability, and performance challenges that remain for fuel cell technology. In Fuel Cell Chemistry and Operation ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 1–13. [Google Scholar]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- He, C.; Desai, S.; Brown, G.; Bollepalli, S. PEM Fuel Cell Catalysts: Cost, Performance, and Durability. Electrochem. Soc. Interface 2005, 14, 41–44. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Narayanasamy, M. Ultra-low loading of platinum in proton exchange membrane-based fuel cells: A brief review. Mater. Renew. Sustain. Energy 2019, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Zou, J.; Mukerjee, S.; Liu, Z.; Yang, D.; Zhang, Y.; Peng, Y.; Dong, A. Ultralow platinum loading proton exchange membrane fuel cells: Performance losses and solutions. J. Power Sources 2021, 490, 229515. [Google Scholar] [CrossRef]
- Bonakdarpour, A.; Dahn, T.R.; Atanasoski, R.T.; Debe, M.K.; Dahn, J.R. H2O2 release during oxygen reduction reaction on Pt nanoparticles. Electrochem. Solid-State Lett. 2008, 11, 208–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An Overview on Metal Oxide Materials as Electrocatalysts and Supports for Polymer Electrolyte Fuel Cells Zhonghua. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Chen, Y.; Zhang, J.; Luo, Y.; Wang, G.; Wang, R. An enhanced activity of Pt/CeO2/CNT triple junction interface catalyst prepared by atomic layer deposition for oxygen reduction reaction. Chem. Phys. Lett. 2020, 755, 137793. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Easton, E.B. Exceptionally durable Pt/TOMS catalysts for fuel cells. Appl. Catal. B Environ. 2020, 268, 118743. [Google Scholar] [CrossRef]
- Alipour MoghadamEsfahani, R.; Vankova, S.K.; Easton, E.B.; Ebralidze, I.I.; Specchia, S. A hybrid Pt/NbO/CNTs catalyst with high activity and durability for oxygen reduction reaction in PEMFC. Renew. Energy 2020, 154, 913–924. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.; Sa, B.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for improving the ORR activity and durability of PEMFC. Int. J. Hydrogen Energy 2017, 42, 13011–13019. [Google Scholar] [CrossRef]
- Meng, C.; Ling, T.; Ma, T.Y.; Wang, H.; Hu, Z.; Zhou, Y.; Mao, J.; Du, X.W.; Jaroniec, M.; Qiao, S.Z. Atomically and Electronically Coupled Pt and CoO Hybrid Nanocatalysts for Enhanced Electrocatalytic Performance. Adv. Mater. 2017, 29, 1604607. [Google Scholar] [CrossRef]
- Sasaki, K.; Zhang, L.; Adzic, R.R. Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction. Phys. Chem. Chem. Phys. 2008, 10, 159–167. [Google Scholar] [CrossRef]
- Chalgin, A.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Wu, J. Effect of supporting materials on the electrocatalytic activity, stability and selectivity of noble metal-based catalysts for oxygen reduction and hydrogen evolution reactions. Prog. Nat. Sci. Mater. Int. 2020, 30, 289–297. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Popov, B.N. Electrocatalytic activity and stability of titania-supported platinum-palladium electrocatalysts for polymer electrolyte membrane fuel cell. ACS Catal. 2012, 2, 825–831. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhao, L.; Zhang, C.; Yan, Y.; Xian, Y. Controlled growth cerium oxide nanoparticles on reduced graphene oxide for oxygen catalytic reduction. Electrochim. Acta 2016, 191, 669–676. [Google Scholar] [CrossRef]
- Spiel, C.; Blaha, P.; Suchorski, Y.; Schwarz, K.; Rupprechter, G. CeO2/Pt(111) interface studied using first-principles density functional theory calculations. Phys. Rev. B-Condens. Matter Mater. Phys. 2011, 84, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen defects and surface chemistry of ceria: Quantum chemical studies compared to experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, L.; Yang, C.; Yuan, Y. CeO2 nanoparticle-decorated reduced graphene oxide as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Int. J. Hydrogen Energy 2017, 42, 15140–15148. [Google Scholar] [CrossRef]
- Łańcucki, Ł.; Kruczała, K. Development of stabilized protonconducting membrane based on poly(ethylene-co-vinyl alcohol). Polym. Degrad. Stab. 2014, 109, 327–335. [Google Scholar] [CrossRef]
- Schlick, S.; Danilczuk, M.; Drews, A.R.; Kukreja, R.S. Scavenging of Hydroxyl Radicals by Ceria Nanoparticles: Effect of Particle Size and Concentration. J. Phys. Chem. C 2016, 120, 6885–6890. [Google Scholar] [CrossRef]
- Danilczuk, M.; Schlick, S.; Coms, F.D. Cerium(III) as a Stabilizer of Perfluorinated Membranes Used in Fuel Cells: In Situ Detection of Early Events in the ESR Resonator. Macromolecules 2009, 42, 8943–8949. [Google Scholar] [CrossRef]
- Baker, A.M.; Stewart, S.M.; Ramaiyan, K.P.; Banham, D.; Ye, S.; Garzon, F.; Mukundan, R.; Borup, R.L. Doped Ceria Nanoparticles with Reduced Solubility and Improved Peroxide Decomposition Activity for PEM Fuel Cells. J. Electrochem. Soc. 2021, 168, 24507. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalyst and Catalyst Layers. Fundamentals and Applications; Springer: London, UK, 2008; pp. 89–134. ISBN 978-1-84800-935-6. [Google Scholar]
- Fugane, K.; Mori, T.; Ou, D.R.; Suzuki, A.; Yoshikawa, H.; Masuda, T.; Uosaki, K.; Yamashita, Y.; Ueda, S.; Kobayashi, K.; et al. Activity of oxygen reduction reaction on small amount of amorphous CeO x promoted Pt cathode for fuel cell application. Electrochim. Acta 2011, 56, 3874–3883. [Google Scholar] [CrossRef]
- Lim, D.H.; Lee, W.D.; Choi, D.H.; Lee, H.I. Effect of ceria nanoparticles into the Pt/C catalyst as cathode material on the electrocatalytic activity and durability for low-temperature fuel cell. Appl. Catal. B Environ. 2010, 94, 85–96. [Google Scholar] [CrossRef]
- Xu, H.; Hou, X. Synergistic effect of CeO2 modified Pt/C electrocatalysts on the performance of PEM fuel cells. Int. J. Hydrogen Energy 2007, 32, 4397–4401. [Google Scholar] [CrossRef]
- Masuda, T.; Fukumitsu, H.; Fugane, K.; Togasaki, H.; Matsumura, D.; Tamura, K.; Nishihata, Y.; Yoshikawa, H.; Kobayashi, K.; Mori, T.; et al. Role of cerium oxide in the enhancement of activity for the oxygen reduction reaction at Pt-CeOx nanocomposite electrocatalyst—An in situ electrochemical X-ray absorption fine structure study. J. Phys. Chem. C 2012, 116, 10098–10102. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Wang, S.; Sun, G. Durable Platinum-Based Electrocatalyst Supported by Multiwall Carbon Nanotubes Modified with CeO2. ChemElectroChem 2018, 5, 2442–2448. [Google Scholar] [CrossRef]
- Luo, Y.; Calvillo, L.; Daiguebonne, C.; Daletou, M.K.; Granozzi, G.; Alonso-Vante, N. A highly efficient and stable oxygen reduction reaction on Pt/CeOx/C electrocatalyst obtained via a sacrificial precursor based on a metal-organic framework. Appl. Catal. B Environ. 2016, 189, 39–50. [Google Scholar] [CrossRef]
- Mohan, N.; Cindrella, L. Template-free synthesis of Pt-MOx (M = Ni, Co & Ce) supported on cubic zeolite-A and their catalytic role in methanol oxidation and oxygen reduction reactions characterized by the hydrodynamic study. Int. J. Hydrogen Energy 2017, 42, 21719–21731. [Google Scholar] [CrossRef]
- Kong, F.D.; Yin, G.P.; Du, C.Y.; Zhang, S.; Qu, Y.T.; Du, L.; Xu, Z.Q.; Ling, A.X. 3D-niobium oxide supported platinum as an effective and durable oxygen reduction catalyst. Catal. Commun. 2015, 68, 67–72. [Google Scholar] [CrossRef]
- Senevirathne, K.; Hui, R.; Campbell, S.; Ye, S.; Zhang, J. Electrocatalytic activity and durability of Pt/NbO2 and Pt/Ti4O7 nanofibers for PEM fuel cell oxygen reduction reaction. Electrochim. Acta 2012, 59, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, L.; Holt, C.M.B.; Navessin, T.; Malek, K.; Eikerling, M.H.; Mitlin, D. Oxygen reduction reaction activity and electrochemical stability of thin-film bilayer systems of platinum on niobium oxide. J. Phys. Chem. C 2010, 114, 16463–16474. [Google Scholar] [CrossRef] [Green Version]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Jia, Q.; Ghoshal, S.; Li, J.; Liang, W.; Meng, G.; Che, H.; Zhang, S.; Ma, Z.F.; Mukerjee, S. Metal and Metal Oxide Interactions and Their Catalytic Consequences for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2017, 139, 7893–7903. [Google Scholar] [CrossRef]
- Ando, F.; Tanabe, T.; Gunji, T.; Tsuda, T.; Kaneko, S.; Takeda, T.; Ohsaka, T.; Matsumoto, F. Improvement of ORR Activity and Durability of Pt Electrocatalyst Nanoparticles Anchored on TiO2/Cup-Stacked Carbon Nanotube in Acidic Aqueous Media. Electrochim. Acta 2017, 232, 404–413. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Santoyo, H.H.R.; Medina-Flores, J.M.; Martínez-Álvarez, O. Platinum deposited on TiO2-C and SnO2-C composites for methanol oxidation and oxygen reduction. Electrochim. Acta 2014, 120, 344–349. [Google Scholar] [CrossRef]
- Kim, D.S.; Zeid, E.F.A.; Kim, Y.T. Additive treatment effect of TiO2 as supports for Pt-based electrocatalysts on oxygen reduction reaction activity. Electrochim. Acta 2010, 55, 3628–3633. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Videla, A.H.A.M.; Vankova, S.; Specchia, S. Stable and methanol tolerant Pt/TiOx-C electrocatalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2015, 40, 14529–14539. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Lakshmi, N.; Dhathathreyan, K.S. Nano titanium oxide catalyst support for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2008, 33, 7521–7526. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Garcia, J.R.V.; Estrada, E.A. Comparison of TiO2 and TiO2 -CNT as Cathode Catalyst Supports for ORR. Int. J. Electrochem. Sci. 2013, 8, 12780–12800. [Google Scholar]
- He, C.; Sankarasubramanian, S.; Matanovic, I.; Atanassov, P.; Ramani, V. Understanding the Oxygen Reduction Reaction Activity and Oxidative Stability of Pt Supported on Nb-Doped TiO2. ChemSusChem 2019, 12, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Karlberg, G.S.; Jaramillo, T.F.; Norskov, J.K. Steady state oxygen reduction and cyclic voltammetry. Faraday Discuss. 2008, 140, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Rähn, M.; Matisen, L.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Pt nanoparticles sputter-deposited on TiO2/MWCNT composites prepared by atomic layer deposition: Improved electrocatalytic activity towards the oxygen reduction reaction and durability in acid media. Int. J. Hydrogen Energy 2018, 43, 4967–4977. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Vankova, S.K.; Monteverde Videla, A.H.A.; Specchia, S. Innovative carbon-free low content Pt catalyst supported on Mo-doped titanium suboxide (Ti3O5-Mo) for stable and durable oxygen reduction reaction. Appl. Catal. B Environ. 2017, 201, 419–429. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Rivera Gavidia, L.M.; García, G.; Pastor, E.; Specchia, S. Highly active platinum supported on Mo-doped titanium nanotubes suboxide (Pt/TNTS-Mo) electrocatalyst for oxygen reduction reaction in PEMFC. Renew. Energy 2018, 120, 209–219. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, G.; Lim, H.; Zhu, C.; You, H.; Kim, Y.T. Effects of transition metal doping in Pt/M-TiO2 (M = V, Cr, and Nb) on oxygen reduction reaction activity. J. Power Sources 2016, 320, 188–195. [Google Scholar] [CrossRef]
- Beauger, C.; Testut, L.; Berthon-Fabry, S.; Georgi, F.; Guetaz, L. Doped TiO2 aerogels as alternative catalyst supports for proton exchange membrane fuel cells: A comparative study of Nb, v and Ta dopants. Microporous Mesoporous Mater. 2016, 232, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, A.; Wu, C.; Nagai, T.; Ohara, K.; Nakada, K.; Matsuzawa, K.; Napporn, T.; Arao, M.; Kuroda, Y.; Tominaka, S.; et al. Factors affecting oxygen reduction activity of Nb2O5-doped TiO2 using carbon nanotubes as support in acidic solution. Electrochim. Acta 2018, 283, 1779–1788. [Google Scholar] [CrossRef]
- Chevallier, L.; Bauer, A.; Cavaliere, S.; Hui, R.; Rozière, J.; Jones, D.J. Mesoporous nanostructured Nb-doped titanium dioxide microsphere catalyst supports for PEM fuel cell electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1752–1759. [Google Scholar] [CrossRef]
- Ishihara, A.; Ohgi, Y.; Matsuzawa, K.; Mitsushima, S.; Ota, K.I. Progress in non-precious metal oxide-based cathode for polymer electrolyte fuel cells. Electrochim. Acta 2010, 55, 8005–8012. [Google Scholar] [CrossRef]
- Sebastián, D.; Baglio, V.; Sun, S.; Tavares, A.C.; Aricò, A.S. Facile synthesis of Zr- and Ta-based catalysts for the oxygen reduction reaction. Chinese J. Catal. 2015, 36, 484–489. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Wang, H.; Wang, Q.; Key, J.; Linkov, V.; Ji, S. Nitrogen-doped carbon coated ZrO2 as a support for Pt nanoparticles in the oxygen reduction reaction. Int. J. Hydrogen Energy 2013, 38, 5783–5788. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Cheng, N.; Peng, T.; Pan, M.; Mu, S. High stability platinum electrocatalysts with zirconia-carbon hybrid supports. J. Mater. Chem. 2012, 22, 1135–1141. [Google Scholar] [CrossRef]
- Mittermeier, T.; Madkikar, P.; Wang, X.; Gasteiger, H.A.; Piana, M. ZrO2 Based Oxygen Reduction Catalysts for PEMFCs: Towards a Better Understanding. J. Electrochem. Soc. 2016, 163, F1543–F1552. [Google Scholar] [CrossRef] [Green Version]
- Sobańska, K.; Pietrzyk, P.; Sojka, Z. Generation of Reactive Oxygen Species via Electroprotic Interaction of H2O2 with ZrO2 Gel: Ionic Sponge Effect and pH-Switchable Peroxidase- and Catalase-Like Activity. ACS Catal. 2017, 7, 2935–2947. [Google Scholar] [CrossRef]
- Baturina, O.; Garsany, Y.; Zega, T.; Stroud, R.; Swider-Lyons, K. Oxygen Reduction Reaction on Platinum/Tantalum Oxide Electrocatalysts for PEM Fuel Cells. J. Electrochem. Soc. 2008, 155, B1314–B1321. [Google Scholar] [CrossRef]
- Rutkowska, I.A.; Wadas, A.; Zoladek, S.; Skunik-Nuckowska, M.; Miecznikowski, K.; Negro, E.; Noto, V.D.; Zlotorowicz, A.; Zelenay, P.; Kulesza, P.J. Activation of Reduced-Graphene-Oxide Supported Pt Nanoparticles by Aligning with WO3 -Nanowires toward Oxygen Reduction in Acid Medium: Diagnosis with Rotating-Ring-Disk Voltammetry and Double-Potential-Step Chronocoulometry. J. Electrochem. Soc. 2018, 165, J3384–J3391. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Z.; Dou, M.; Wang, F. Highly Dispersed and Crystalline Ta2O5 Anchored Pt Electrocatalyst with Improved Activity and Durability Toward Oxygen Reduction: Promotion by Atomic-Scale Pt-Ta2O5 Interactions. ACS Catal. 2019, 9, 3278–3288. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Sun, S. Structurally ordered FePt nanoparticles and their enhanced catalysis for oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 4996–4997. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Synthesis and oxygen reduction electrocatalytic property of Pt-on-Pd bimetallic heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Peng, Z.; Yang, S.; Wagner, F.T. Truncated Octahedral Pt3Ni Oxygen Reduction Reaction Electrocatalysts. J. Am. Chem. Soc. 2010, 132, 4984–4985. [Google Scholar] [CrossRef]
- Sasaki, K.; Naohara, H.; Choi, Y.; Cai, Y.; Chen, W.F.; Liu, P.; Adzic, R.R. Highly stable Pt monolayer on PdAu nanoparticle electrocatalysts for the oxygen reduction reaction. Nat. Commun. 2012, 3, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Chen, Z.; Huang, S. Rapid synthesis of hollow PtPdCu trimetallic octahedrons at room temperature for oxygen reduction reactions in acid media. CrystEngComm 2020, 22, 1586–1592. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface 2015, 24, 45–49. [Google Scholar] [CrossRef]
- Negro, E.; Polizzi, S.; Vezzù, K.; Toniolo, L.; Cavinato, G.; Di Noto, V. Interplay between morphology and electrochemical performance of “core-shell” electrocatalysts for oxygen reduction reaction based on a PtNix carbon nitride “shell” and a pyrolyzed polyketone nanoball “core”. Int. J. Hydrogen Energy 2014, 39, 2828–2841. [Google Scholar] [CrossRef] [Green Version]
- Di Noto, V.; Negro, E. Pt-Fe and Pt-Ni carbon nitride-based “core-shell” ORR electrocatalysts for polymer electrolyte membrane fuel cells. Fuel Cells 2010, 10, 234–244. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Polizzi, S.; Vezzù, K.; Toniolo, L.; Cavinato, G. Synthesis, studies and fuel cell performance of “core-shell” electrocatalysts for oxygen reduction reaction based on a PtNix carbon nitride “shell” and a pyrolyzed polyketone nanoball “core”. Int. J. Hydrogen Energy 2014, 39, 2812–2827. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolini, E.; Salgado, J.R.C.; Giz, M.J.; Gonzalez, E.R. Effects of geometric and electronic factors on ORR activity of carbon supported Pt-Co electrocatalysts in PEM fuel cells. Int. J. Hydrogen Energy 2005, 30, 1213–1220. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chemie-Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. The stability of Pt-M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells. A literature review and tests on a Pt-Co catalyst. J. Power Sources 2006, 160, 957–968. [Google Scholar] [CrossRef]
- Colón-Mercado, H.R.; Popov, B.N. Stability of platinum based alloy cathode catalysts in PEM fuel cells. J. Power Sources 2006, 155, 253–263. [Google Scholar] [CrossRef]
- Bonakdarpour, A.; Wenzel, J.; Stevens, D.A.; Sheng, S.; Monchesky, T.L.; Löbel, R.; Atanasoski, R.T.; Schmoeckel, A.K.; Vernstrom, G.D.; Debe, M.K.; et al. Studies of Transition Metal Dissolution from Combinatorially Sputtered, Nanostructured Pt1−xMx (M = Fe, Ni; 0 < x < 1) Electrocatalysts for PEM Fuel Cells. J. Electrochem. Soc. 2005, 152, A61. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Wu, Z.; Ruan, M.; Song, P.; Jiang, L.; Wang, Y.; Sun, G.; Xu, W. Pt0.61Ni/C for High-Efficiency Cathode of Fuel Cells with Superhigh Platinum Utilization. J. Phys. Chem. C 2018, 122, 14691–14697. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Fang, J.; Zou, S. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ren, Z.; Norouzi Banis, M.; Du, L.; Zhou, X.; Chen, G.; Zhang, L.; Li, J.; Wang, S.; Li, M.; et al. Active and Stable Pt-Ni Alloy Octahedra Catalyst for Oxygen Reduction via Near-Surface Atomical Engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Choi, S.-I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Shin, H.; Kim, M.; Lee, H.; Lee, K.S.; Kwon, Y.; Song, D.; Oh, S.; Kim, H.; Cho, E. Ga-Doped Pt-Ni Octahedral Nanoparticles as a Highly Active and Durable Electrocatalyst for Oxygen Reduction Reaction. Nano Lett. 2018, 18, 2450–2458. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Lu, J.; Luo, L.; Qian, G.; Chen, J.; Abbo, H.S.; Titinchi, S.J.J.; Yin, S. Enhancement of oxygen reduction activity and stability via introducing acid-resistant refractory Mo and regulating the near-surface Pt content. J. Energy Chem. 2020, 51, 246–252. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, Z.; Liu, Z.; Gao, W.; Dai, S.; Gha, J.; Xue, W.; Sun, H.; Duan, X.; Pan, X.; et al. Differential Surface Elemental Distribution Leads to Significantly Enhanced Stability of PtNi-Based ORR Catalysts. Matter 2019, 1, 1567–1580. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, W.; Lin, A.; Cheng, D. Low Pt-Content Ternary PtNiCu Nanoparticles with Hollow Interiors and Accessible Surfaces as Enhanced Multifunctional Electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 9600–9608. [Google Scholar] [CrossRef]
- Jia, Q.; Zhao, Z.; Cao, L.; Li, J.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.; Li, M.; et al. Roles of Mo Surface Dopants in Enhancing the ORR Performance of Octahedral PtNi Nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Beermann, V.; Gocyla, M.; Willinger, E.; Rudi, S.; Heggen, M.; Dunin-Borkowski, R.E.; Willinger, M.G.; Strasser, P. Rh-Doped Pt-Ni Octahedral Nanoparticles: Understanding the Correlation between Elemental Distribution, Oxygen Reduction Reaction, and Shape Stability. Nano Lett. 2016, 16, 1719–1725. [Google Scholar] [CrossRef]
- Huang, X.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; Duan, X.; et al. High-performance transition metal – doped Pt 3 Ni octahedra for oxygen reduction reaction. Science (80-.). 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [Green Version]
- Dionigi, F.; Weber, C.C.; Primbs, M.; Gocyla, M.; Bonastre, A.M.; Spöri, C.; Schmies, H.; Hornberger, E.; Kühl, S.; Drnec, J.; et al. Controlling Near-Surface Ni Composition in Octahedral PtNi(Mo) Nanoparticles by Mo Doping for a Highly Active Oxygen Reduction Reaction Catalyst. Nano Lett. 2019, 19, 6876–6885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-I.; Choi, R.; Han, S.W.; Park, J.T. Shape-controlled synthesis of Pt3Co nanocrystals with high electrocatalytic activity toward oxygen reduction. Chem.-A Eur. J. 2011, 17, 12280–12284. [Google Scholar] [CrossRef] [PubMed]
- Chaisubanan, N.; Chanlek, N.; Puarporn, Y.; Limphirat, W.; Piumsomboon, P.; Pruksathorn, K.; Hunsom, M. Insight into the alternative metal oxide modified carbon-supported PtCo for oxygen reduction reaction in proton exchange membrane fuel cell. Renew. Energy 2019, 139, 679–687. [Google Scholar] [CrossRef]
- Jung, W.S.; Lee, W.H.; Oh, H.S.; Popov, B.N. Highly stable and ordered intermetallic PtCo alloy catalyst supported on graphitized carbon containing Co@CN for oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 19833–19842. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Nale, A.; Kulesza, P.J.; Rutkowska, I.A.; Vezzù, K.; Pagot, G. Correlation between Precursor Properties and Performance in the Oxygen Reduction Reaction of Pt and Co “Core-shell” Carbon Nitride-Based Electrocatalysts. Electrocatalysis 2020, 11, 143–159. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of Platinum Oxygen-Reduction Electrocatalysts Using Gold Clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.L.; Miller, J.T.; Guo, N.; Heck, K.N.; Alvarez, P.J.J.; Wong, M.S. Structural analysis of palladium-decorated gold nanoparticles as colloidal bimetallic catalysts. Catal. Today 2011, 160, 96–102. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon N. Y. 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheon, J.Y.; Shin, T.J.; Park, J.Y.; Joo, S.H. Effect of surface oxygen functionalization of carbon support on the activity and durability of Pt/C catalysts for the oxygen reduction reaction. Carbon N. Y. 2016, 101, 449–457. [Google Scholar] [CrossRef]
- Wang, D.W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC. Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wilkinson, D.P.; Zhang, J. Noncarbon support materials for polymer electrolyte membrane fuel cell electrocatalysts. Chem. Rev. 2011, 111, 7625–7651. [Google Scholar] [CrossRef]
- Xue, Q.; Yang, D.; Jiang, L.; Li, B.; Ming, P. Modifying Carbon Supports of Catalyst for the Oxygen Reduction Reaction in Vehicle PEMFCs. Automot. Innov. 2021, 4, 119–130. [Google Scholar] [CrossRef]
- Padgett, E.; Yarlagadda, V.; Holtz, M.E.; Ko, M.; Levin, B.D.A.; Kukreja, R.S.; Ziegelbauer, J.M.; Andrews, R.N.; Ilavsky, J.; Kongkanand, A.; et al. Mitigation of PEM Fuel Cell Catalyst Degradation with Porous Carbon Supports. J. Electrochem. Soc. 2019, 166, F198–F207. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR. A critical assessment about the role of heteroatoms. Carbon N. Y. 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and Activation of O2 on Nitrogen-Doped Carbon Nanotubes Xingbang. J. Phys. Chem. C 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Zhang, H.; Tian, W.; Tan, Y.; Qian, W.; Liu, Z. Low content Pt nanoparticles anchored on N-doped reduced graphene oxide with high and stable electrocatalytic activity for oxygen reduction reaction. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jukk, K.; Kongi, N.; Rauwel, P.; Matisen, L.; Tammeveski, K. Platinum Nanoparticles Supported on Nitrogen-Doped Graphene Nanosheets as Electrocatalysts for Oxygen Reduction Reaction. Electrocatalysis 2016, 7, 428–440. [Google Scholar] [CrossRef]
- Ma, J.; Habrioux, A.; Luo, Y.; Ramos-Sanchez, G.; Calvillo, L.; Granozzi, G.; Balbuena, P.B.; Alonso-Vante, N. Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: Effect on the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 11891–11904. [Google Scholar] [CrossRef]
- Galeano, C.; Meier, J.C.; Soorholtz, M.; Bongard, H.; Baldizzone, C.; Mayrhofer, K.J.J.; Schüth, F. Nitrogen-doped hollow carbon spheres as a support for platinum-based electrocatalysts. ACS Catal. 2014, 4, 3856–3868. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Wang, F. Low-loading Pt nanoparticles embedded on Ni, N-doped carbon as superior electrocatalysts for oxygen reduction. Catal. Sci. Technol. 2020, 10, 65–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Xuan, W.; Wang, W.; Ding, W.; Wei, Z. ZIF derived mesoporous carbon frameworks with numerous edges and heteroatom-doped sites to anchor nano-Pt electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 22649–22657. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, S.G.; Han, S.B.; Park, K.W. Synergistically Enhanced Electrocatalytic Stability of Pt Catalyst Supported by Doped Porous Carbon Nanostructure for Oxygen Reduction Reaction. Electrocatalysis 2020, 11, 497–504. [Google Scholar] [CrossRef]
- Denis, P.A. Band gap opening of monolayer and bilayer graphene doped with aluminium, silicon, phosphorus, and sulfur. Chem. Phys. Lett. 2010, 492, 251–257. [Google Scholar] [CrossRef]
- Denis, P.A.; Faccio, R.; Mombru, A.W. Is it possible to dope single-walled carbon nanotubes and graphene with sulfur? ChemPhysChem 2009, 10, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, L.; Li, J.; Ding, W.; Wei, Z. Modulating the oxygen reduction activity of heteroatom-doped carbon catalysts via the triple effect: Charge, spin density and ligand effect. Chem. Sci. 2018, 9, 5795–5804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, K.; Chung, S.; Lee, J. Narrow size distribution of Pt nanoparticles covered by an S-doped carbon layer for an improved oxygen reduction reaction in fuel cells. J. Power Sources 2020, 450, 227650. [Google Scholar] [CrossRef]
- Yao, R.; Gu, J.; He, H.; Yu, T. Improved Electrocatalytic Activity and Durability of Pt Nanoparticles Supported on Boron-Doped Carbon Black. Catalysts 2020, 10, 862. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, Y.; Ionescu, M.I.; Li, R.; Sun, X.; Ye, S.; Knights, S. 3D boron doped carbon nanorods/carbon-microfiber hybrid composites: Synthesis and applications in a highly stable proton exchange membrane fuel cell. J. Mater. Chem. 2011, 21, 18195–18198. [Google Scholar] [CrossRef]
- Acharya, C.; Turner, H. Stabilization of Platinum Clusters by Substitutional Boron Dopants in Carbon Supports. J. Phys. Chem. B 2006, 110, 17706–17710. [Google Scholar] [CrossRef]
- Li, Y.H.; Hung, T.H.; Chen, C.W. A first-principles study of nitrogen- and boron-assisted platinum adsorption on carbon nanotubes. Carbon N. Y. 2009, 47, 850–855. [Google Scholar] [CrossRef]
- Wu, X.; Radovic, L.R. Inhibition of catalytic oxidation of carbon/carbon composites by phosphorus. Carbon N. Y. 2006, 44, 141–151. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of the oxidation resistance of phosphorus-containing activated carbons. Carbon N. Y. 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Bandosz, T.J. Surface Chemistry of Carbon Materials. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 45–92. [Google Scholar]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon N. Y. 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Q.; Zhang, R.; Wang, Q.; Kang, G.; Peng, F. Phosphorus-doped carbon nanotubes supported low Pt loading catalyst for the oxygen reduction reaction in acidic fuel cells. J. Power Sources 2014, 268, 171–175. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostuch, A.; Rutkowska, I.A.; Dembinska, B.; Wadas, A.; Negro, E.; Vezzù, K.; Di Noto, V.; Kulesza, P.J. Enhancement of Activity and Development of Low Pt Content Electrocatalysts for Oxygen Reduction Reaction in Acid Media. Molecules 2021, 26, 5147. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26175147
Kostuch A, Rutkowska IA, Dembinska B, Wadas A, Negro E, Vezzù K, Di Noto V, Kulesza PJ. Enhancement of Activity and Development of Low Pt Content Electrocatalysts for Oxygen Reduction Reaction in Acid Media. Molecules. 2021; 26(17):5147. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26175147
Chicago/Turabian StyleKostuch, Aldona, Iwona A. Rutkowska, Beata Dembinska, Anna Wadas, Enrico Negro, Keti Vezzù, Vito Di Noto, and Pawel J. Kulesza. 2021. "Enhancement of Activity and Development of Low Pt Content Electrocatalysts for Oxygen Reduction Reaction in Acid Media" Molecules 26, no. 17: 5147. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26175147