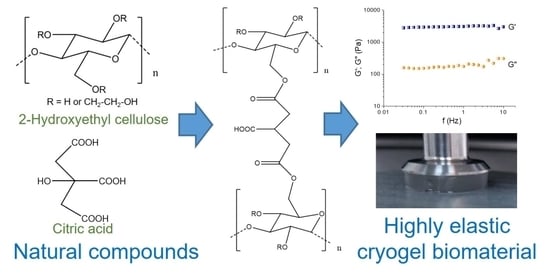
Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Cryogels
2.2. Influence of CA Concentration, Reaction Temperature, and Reaction Time on the Gel Fraction Yield
2.3. Swelling Degree
2.4. Interior Morphology
2.5. Dynamic Rheological Properties
2.6. Hydrolytic Degradation
3. Discussion
4. Materials and Methods
4.1. Compounds and Reagents
4.2. Synthesis of Cryogels
4.3. Determination of Gel Fraction Yield and Swelling Degree
4.4. Freeze Drying
4.5. Rheological Measurements
4.6. Scanning Electron Microscopy
4.7. Hydrolytic Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A


References
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Romain, C.; Williams, C. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purwar, R.; Srivastava, C.M. Antimicrobial cellulose and cellulose derivative materials. In Cellulose and Cellulose Derivatives: Synthesis, Modification and Applications; Mondal, M.I.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 455–472. [Google Scholar]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-Based Biomimetics and Their Applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, Q.; Zhong, Q.; Liu, L.; Meng, R.; Yao, J. Macroporous cellulose-based cryogels with tunable porous structure and surface functional groups. Fibers Polym. 2016, 17, 712–720. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Hasanin, M.; Youssef, A.M.; Aldalbahi, A.; El-Newehy, M.H.; Abdelhameed, R.M. Hydroxyethyl cellulose/bacterial cellulose cryogel dopped silver@titanium oxide nanoparticles: Antimicrobial activity and controlled release of Tebuconazole fungicide. Int. J. Biol. Macromol. 2020, 165, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.; Petrova, E.; Stamenova, R.; Tsvetanov, C.B.; Riess, G. Cryogels of cellulose derivatives prepared via UV irradiation of moderately frozen systems. Polymer 2006, 47, 6481–6484. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, E.; Tchorbanov, B.; Tsvetanov, C.B. Synthesis of biodegradable hydroxyethylcellulose cryogels by UV irradiation. Polymer 2007, 48, 4943–4949. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, E.; Tsvetanov, C.B. UV-assisted synthesis of super-macroporous polymer hydrogels. Polymer 2009, 50, 1118–1123. [Google Scholar] [CrossRef]
- Stoyneva, V.; Momekova, D.; Kostova, B.; Petrov, P. Stimuli sensitive super-macroporous cryogels based on photo-crosslinked 2-hydroxyethylcellulose and chitosan. Carbohydr. Polym. 2014, 99, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohydr. Polym. 2011, 85, 664–673. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Lyagin, I.V.; Lozinsky, V.I. Enzymatic biocatalysts immobilized on/in the cryogel-type carriers. In Supermacroporous Cryogels; Kumar, A., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 311–334. [Google Scholar]
- Ivanova, J.G.; Kabaivanova, L.V.; Petrov, P.D.; Yankova, S.N. Optimization strategies for improved growth, polysaccharide production and storage of the red microalga Rhodella reticulata. Bulg. Chem. Commun. 2015, 47, 167–174. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozova, N.; Petrov, P.D. Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State. Molecules 2021, 26, 6370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216370
Bozova N, Petrov PD. Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State. Molecules. 2021; 26(21):6370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216370
Chicago/Turabian StyleBozova, Nadegda, and Petar D. Petrov. 2021. "Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State" Molecules 26, no. 21: 6370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216370