Design and Synthesis of (2-oxo-1,2-Dihydroquinolin-4-yl)-1,2,3-triazole Derivatives via Click Reaction: Potential Apoptotic Antiproliferative Agents
Abstract
:1. Introduction

2. Results
2.1. Chemistry Section
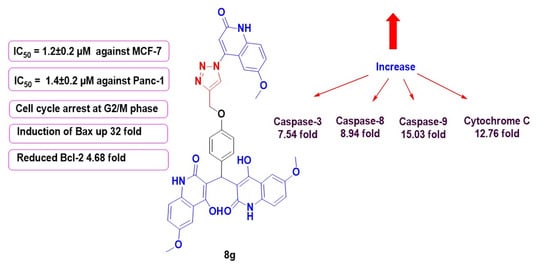
2.2. Pharmacological Assays
2.2.1. Cell Viability Assay
2.2.2. Cytotoxic Activity and Evaluation of IC50
2.2.3. Apoptosis Assay
Activation of Proteolytic Caspases Cascade
Cytochrome C Assay
Bax and Bcl-2 Levels Assay
Flow Cytometric Cell Cycle Analysis
3. Conclusions
4. Experimental
4.1. Chemistry
4.1.1. Starting Materials
4.1.2. General Procedure for the Synthesis of Compounds 4a–c
4.1.3. General Procedure for the Synthesis of Compounds 8a–l
4.2. Pharmacological Assays
4.2.1. Cytotoxic Activity Using MTT Assay and Evaluation of IC50
MTT Assay
Assay for the Anti-Proliferative Effect
4.2.2. Caspase 3 Activation Assay
4.2.3. Caspase 8 Activation Assay
4.2.4. Bax Activation Assay
4.2.5. Bcl-2 Inhibition Assay
4.2.6. Cytochrome C Assay
4.2.7. Cell Apoptosis Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Batista, V.F.; Pinto, D.C.; Silva, A.M. Synthesis of quinolines: A green perspective. ACS Sustain. Chem. Eng. 2016, 4, 4064. [Google Scholar] [CrossRef]
- Dhiman, P.; Arora, N.; Thanikachalam, P.V.; Monga, V. Recent advances in the synthetic and medicinal perspective of quinolones. A review. Bioorg. Chem. 2019, 92, 103291. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ramadan, M.; Abuo-Rahma, G.E.-D.A.; Elshaier, Y.A.; Elbastawesy, M.A.; Brown, A.B.; Bräse, S. Quinolones as prospective drugs: Their syntheses and biological applications. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, X.; Wang, T.; Xiao, J. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019, 165, 59. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Banerjee, K.; Chakraborty, P.; Das, S.; Sarkar, A.; Hazra, A.; Banerjee, M.; Maity, A.; Chatterjee, M.; Mondal, N.B. Overcoming multidrug resistance (MDR) in cancer in vitro and in vivo by a quinoline derivative. Biomed. Pharmacother. 2011, 65, 387. [Google Scholar] [CrossRef]
- Elbastawesy, M.A.; Aly, A.A.; Ramadan, M.; Elshaier, Y.A.; Youssif, B.G.; Brown, A.B.; Abuo-Rahma, G.E.-D.A. Novel Pyrazoloquinolin-2-ones: Design, synthesis, docking studies, and biological evaluation as anti-proliferative EGFR-TK inhibitors. Bioorg. Chem. 2019, 90, 103045. [Google Scholar] [CrossRef]
- Elbastawesy, M.A.; Ramadan, M.; El-Shaier, Y.A.; Aly, A.A.; Abuo-Rahma, G.E.-D.A. Arylidenes of Quinolin-2-one scaffold as Erlotinib analogues with activities against leukemia through inhibition of EGFR TK/STAT-3 pathways. Bioorg. Chem. 2020, 96, 103628. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Abd El-Aziz, M.; Elshaier, Y.A.; Youssif, B.G.; Brown, A.B.; Fathy, H.M.; Aly, A.A. Design and synthesis of new pyranoquinolinone heteroannulated to triazolopyrimidine of potential apoptotic anti-proliferative activity. Bioorg. Chem. 2020, 105, 104392. [Google Scholar] [CrossRef]
- Sathish Kumar, S.; Kavitha, P.H. Synthesis and biological applications of triazole derivatives: A review. Mini. Rev. Org. Chem. 2013, 10, 40. [Google Scholar] [CrossRef]
- Huang, M.; Deng, Z.; Tian, J.; Liu, T. Synthesis and biological evaluation of salinomycin triazole analogues as anti-cancer agents. Eur. J. Med. Chem. 2017, 127, 900. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30. [Google Scholar] [CrossRef]
- Xu, J.H.; Fan, Y.L.; Zhou, J. Quinolone-Triazole Hybrids and their Biological Activities. Heterocycl. Chem. 2018, 55, 1854. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1,2,4-Triazole-quinoline/quinolone hybrids as potential antibacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nandivada, H.; Jiang, X.; Lahann, J. Click chemistry: Versatility and control in the hands of materials scientists. Adv. Mater. 2007, 19, 2197. [Google Scholar] [CrossRef]
- Gehringer, M.; Laufer, S.A. Click chemistry: Novel applications in cell biology and drug discovery. Angew. Chem. Int. Ed. 2017, 56, 15504. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.; Mourad, M.A.; Bräse, S.; Ibrahim, M.A.; Nieger, M.; Garvalov, B.K.; Dalby, K.N.; Kaoud, T.S. Design, synthesis and biological evaluation of fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinoline-6,7,8,13-tetraones derivatives as ERK inhibitors with efficacy in BRAF-mutant melanoma. Bioorg. Chem. 2019, 82, 290. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.; Mourad, M.A.; Brown, A.B.; Bräse, S.; Nieger, M.; Ibrahim, M.A. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg. Chem. 2018, 81, 700. [Google Scholar] [CrossRef]
- Elbastawesy, M.A.; El-Shaier, Y.A.; Ramadan, M.; Brown, A.B.; Aly, A.A.; Abuo-Rahma, G.E.-D.A. Identification and molecular modeling of new quinolin-2-one thiosemicarbazide scaffold with antimicrobial urease inhibitory activity. Mol. Divers. 2020, 25, 13. [Google Scholar] [CrossRef]
- Abass, M. Chemistry of substituted quinolinones. Part II synthesis of novel 4-pyrazolylquinolinone derivatives. Synth. Commun. 2000, 30, 2735. [Google Scholar] [CrossRef]
- Ismail, M.M.; Abass, M.; Hassan, M.M. Chemistry of substituted quinolinones. Part VI. Synthesis and nucleophilic reactions of 4-chloro-8-methylquinolin-2(1H)-one and its thione analogue. Molecules 2000, 5, 1224. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.A.; El-Sheref, E.M.; Mourad, A.-F.E.; Bakheet, M.E.; Bräse, S.; Nieger, M. One-pot synthesis of 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl) succinates and arylmethylene-bis(3,3’-quinoline-2-ones). Chem. Pap. 2019, 73, 27. [Google Scholar] [CrossRef] [Green Version]
- Hisham, M.; Youssif, B.G.; Osman, E.E.A.; Hayallah, A.M.; Abdel-Aziz, M. Synthesis and biological evaluation of novel xanthine derivatives as potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 176, 117. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Gouda, A.M.; Abou-Ghadir, O.F.; Salem, O.I.; Ali, A.T.; Farghaly, H.S.; Abdelrahman, M.H.; Trembleau, L.; Abdu-Allah, H.H.; Youssif, B.G. Design and synthesis of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as anti-proliferative EGFR and BRAFV600E dual inhibitors. Bioorg. Chem. 2020, 104, 104260. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.; Mohamed, A.M.; Osman, E.E.A.; Abou-Ghadir, O.F.; Elnaggar, D.H.; Abdelrahman, M.H.; Treamblu, L.; Gomaa, H.A. 5-Chlorobenzofuran-2-carboxamides: From allosteric CB1 modulators to potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 177, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, A.H.; El-Saadi, M.T.; Said, E.G.; Youssif, B.G.; Omar, H.A.; El-Moghazy, S.M. Novel diphenylthiazole derivatives with multi-target mechanism: Synthesis, docking study, anti-cancer and anti-inflammatory activities. Bioorg. Chem. 2017, 75, 127. [Google Scholar] [CrossRef]
- Nguyen, T.; Li, J.X.; Thomas, B.F.; Wiley, J.L.; Kenakin, T.P.; Zhang, Y. Allosteric modulation: An alternate approach targeting the cannabinoid CB1 receptor. Med. Res. Rev. 2017, 37, 441. [Google Scholar] [CrossRef] [Green Version]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1. [Google Scholar] [CrossRef] [Green Version]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and-7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Debatin, K.-M. Extrinsic versus intrinsic apoptosis pathways in anti-cancer chemotherapy. Oncogene 2006, 25, 4798. [Google Scholar] [CrossRef] [Green Version]
- Abdelbaset, M.S.; Abuo-Rahma, G.E.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G.; Bukhari, S.N.A.; Mohamed, M.F.; Abdel-Aziz, M. Novel pyrrol-2(3H)-ones and pyridazin-3(2H)-ones carrying quinoline scaffold as anti-proliferative tubulin polymerization inhibitors. Bioorg. Chem. 2018, 80, 151. [Google Scholar] [CrossRef]
- Abou-Zied, H.A.; Youssif, B.G.; Mohamed, M.F.; Hayallah, A.M.; Abdel-Aziz, M. EGFR inhibitors and apoptotic inducers: Design, synthesis, anti-cancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019, 89, 102997. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.H.; Aboraia, A.S.; Youssif, B.G.; Elsadek, B.M. Design, synthesis and pharmacophoric model building of new 3-alkoxymethyl/3-phenyl indole-2-carboxamides with potential anti-proliferative activity. Chem. Biol. Drug Des. 2017, 90, 64. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G. Novel quinoline derivatives carrying nitrones/oximes nitric oxide donors: Design, synthesis, anti-proliferative and caspase-3 activation activities. Arch. Pharm. 2019, 352, 1800270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuno, T. 4-(Prop-2-yn-1-yloxy) benzaldehyde. Acta Crystall. Sect. E Rep. Online 2013, 69, o125. [Google Scholar]
- Aizikovich, A.; Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Byk, G.; Gellerman, G. A new application of diphenylphosphorylazide (DPPA) reagent: Convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into the 4-azido and 4-amino counterparts. Tetrahedron Lett. 2004, 45, 4241. [Google Scholar] [CrossRef]








| 1H NMR | 1H-1HCOSY | Assignment | |
| 13.39 (bs; 1H) | OH-4/4’ | ||
| 12.90 (bs; 1H) | OH-4’/4 | ||
| 12.19 (bs; 1H) | NH-1/1’ | ||
| 12.08 (bs; 1H) | NH-1’/1 | ||
| 7.40 (d, J = 9.0; 2H) | 7.26 | H-8, 8’ | |
| 7.40 (bs; 1H) | H-5/5’ | ||
| 7.33 (bs; 1H) | H-5’/5 | ||
| 7.26 (bd, J = 8.6; 2H) | 7.40 | H-7, 7’ | |
| 7.02 (d, J = 8.5; 2H) | 6.89 | H-o | |
| 6.89 (d, J = 8.8; 2H) | 7.02 | H-m | |
| 6.14 (s; 1H) | H-a | ||
| 4.76 (d, J = 2.0; 2H) | 3.55 | H-b | |
| 3.77 (s; 6H) | H-6a, 6a’ | ||
| 3.55 (bs; 1H) | 4.76 | H-d | |
| 15N NMR | HSQC | HMBC | Assignment |
| 144.2 | 12.08 | 7.40 | N-1/1’ |
| 142.9 | 12.19 | 7.40 | N-1’/1 |
| 13C NMR | HSQC | HMBC | Assignment |
| 165.76, 164.10 | 6.14 | C-2, 2’ | |
| 161.84, 160.91 | 6.14 | C-4, 4’ | |
| 155.31 | 7.02, 6.89, 4.76 | C-p | |
| 154.80 | 7.40, 7.26, 3.77 | C-6, 6’ | |
| 131.52 | 7.40, 7.26 | C-8a, 8a’ | |
| 130.29 | 6.89, 6.14 | C-i | |
| 127.29 | 7.02 | 7.02, 6.14 | C-o |
| 120.63 | 7.26 | C-7, 7’ | |
| 117.46 | 7.40 | 7.40 | C-8, 8’ |
| 117.21, 116.70 | 7.40, 7.33 | C-4a, 4a’ | |
| 114.35 | 6.89 | 6.89, 6.89 | C-m |
| 111.74, 111.25 | 6.14 | C-3, 3’ | |
| 103.82 | 7.40, 7.33 | C-5, 5’ | |
| 79.43 | 4.76 | C-c | |
| 78.00 | 3.55 | 3.55 | C-d |
| 55.41 | 3.77 | C-6a, 6a’ | |
| 55.34 | 4.76 | C-b | |
| 34.69 | 6.14 | 7.02, 6.14 | C-a |
| 1H NMR | 1H-1HCOSY | Assignment | |
| 13.41 (bs; 1H) | OH-4/4’ | ||
| 12.91 (bs; 1H) | OH-4’/4 | ||
| 12.20 (s; 2H) | 6.81 | NH-1/1’, 1″ | |
| 12.09 (bs; 1H) | NH-1’/1 | ||
| 8.84 (s; 1H) | H-d | ||
| 7.47 (d, J = 8.3; 1H) | 7.38, 7.22 | H-7″ | |
| 7.40 (d, J = 9.2; 2H) | 7.26 | H-8, 8’ | |
| 7.38 (d, J = 8.4; 2H) | 7.47, 7.26, 2.29 | H-5/5’, 8″ | |
| 7.33 (m; 1H) | 7.26 | H-5’/5 | |
| 7.26 (d, J = 8.1; 2H) | 7.40, 7.38, 7.33 | H-7, 7’ | |
| 7.22 (s; 1H) | 7.47, 2.29 | H-5″ | |
| 7.05 (d, J = 8.2; 2H) | 7.01, 6.15 | H-o | |
| 7.01 (d, J = 8.2; 2H) | 7.05 | H-m | |
| 6.81 (s; 1H) | 12.20 | H-3″ | |
| 6.15 (bs; 1H) | 7.05 | H-a | |
| 5.27 (s; 2H) | H-b | ||
| 3.82 (s; 6H) | H-6b, 6b’ | ||
| 2.29 (s; 3H) | 7.40, 7.38, 7.26 | H-6a″ | |
| 13C NMR | HSQC | HMBC | Assignment |
| 165.85, 164.13 | 6.15 | C-2, 2’ | |
| 161.84 | C-4, 4’ | ||
| 160.84 | C-2″, 4″ | ||
| 156.06 | 7.05, 5.27 | C-p | |
| 154.81 | 7.40, 3.82 | C-6, 6’ | |
| 143.42 | 8.84, 7.40, 7.22, 6.81, 5.27 | C-c | |
| 137.50 | 7.47, 7.22 | C-8a″ | |
| 133.15 | 7.47 | 7.47, 7.22 | C-7″ |
| 131.73, 131.51 | 7.38, 7.26, 2.29 | C-8a, 8a’ | |
| 130.07 | C-i | ||
| 127.40 | 7.05 | C-o, 6″ | |
| 126.50 | 8.84 | 8.84,3 7.01, 5.27 | C-d |
| 123.15 | 7.22 | 7.47, 7.05, 2.29 | C-5″ |
| 120.82 | 7.26 | 7.01 | C-7, 7’ |
| 117.69 | 6.81 | 7.05, 6.813 | C-3″ |
| 117.45 | 7.40 | 7.403 | C-8, 8’ |
| 116.69 | 7.38, 7.33 | C-4a, 4a’ | |
| 115.89 | 7.38 | C-8″ | |
| 114.38 | 7.01 | 7.38, 6.81 | C-m, 4a″ |
| 111.86, 110.18 | 6.15 | C-3, 3’ | |
| 103.81 | 7.38, 7.33 | C-5, 5’ | |
| 60.85 | 5.27 | C-b | |
| 55.40 | 3.82 | 3.823 | C-6b, 6b’ |
| 34.71 | 6.15 | C-a | |
| 20.50 | 2.29 | 7.47, 7.22, 2.293 | C-6a″ |
| 15N NMR | HSQC | HMBC | Assignment |
| 247.4 | 8.84, 6.81 | N-e | |
| 151.6 | 12.20 | 7.38, 6.81 | N-1″ |
| 144.2 | 12.09 | N-1’/1 | |
| 142. | 12.20 | N-1/1‘ | |
| N-f, g n/o. | |||
| Comp. | Cell Viability% (50 µM) | Anti-Proliferative Activity IC50 ± SEM (µM) | ||||
|---|---|---|---|---|---|---|
| A-549 | MCF-7 | Panc-1 | HT-29 | Average (IC50) | ||
| 8a | 89 | 5.7 ± 0.4 | 5.3 ± 0.8 | 5.6 ± 0.6 | 5.7 ± 0.4 | 5.575 |
| 8b | 87 | 7.6 ± 0.8 | 6.9 ± 0.8 | 7.6 ± 0.6 | 7.5 ± 0.4 | 7.400 |
| 8c | 91 | 3.9 ± 0.5 | 3.2 ± 0.08 | 4.1 ± 0.2 | 4.2 ± 0.2 | 3.850 |
| 8d | 89 | 12.9 ± 0.8 | 11.5 ± 1.1 | 11.6 ± 0.8 | 11.4 ± 1.2 | 11.850 |
| 8e | 85 | 2.1 ± 0.2 | 1.5 ± 0.7 | 1.7 ± 0.1 | 2.2 ± 0.4 | 1.875 |
| 8f | 87 | 2.9 ± 0.5 | 2.6 ± 0.8 | 3.1 ± 0.2 | 3.4 ± 0.2 | 3.000 |
| 8g | 84 | 1.9 ± 0.2 | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.8 ± 0.4 | 1.575 |
| 8h | 90 | 10.7 ± 2.5 | 10.6 ± 2.9 | 10.8 ± 1.9 | 10.8 ± 1.6 | 10.725 |
| 8i | 90 | 17.9 ± 0.3 | 17.8 ± 1.6 | 17.6 ± 1.5 | 17.9 ± 1.1 | 17.800 |
| 8j | 81 | 22.5 ± 0.2 | 22.1 ± 0.1 | 22.4 ± 0.2 | 22.9 ± 0.6 | 22.474 |
| 8k | 90 | 14.5 ± 2.6 | 13.6 ± 2.2 | 14.6 ± 2.9 | 14.8 ± 1.4 | 14.375 |
| 8l | 96 | 25.3 ± 2.5 | 24.9 ± 1.8 | 24.5 ± 2.3 | 24.2 ± 1.4 | 24.725 |
| Doxorubicin | -- | 1.21 ± 0.80 | 0.90 ± 0.62 | 1.41 ± 0.58 | 1.01 ± 0.82 | 1.136 |
| Compound No. | Caspase 3 | Caspase 8 | Caspase 9 | Cytochrome C | ||||
|---|---|---|---|---|---|---|---|---|
| Conc (pg/mL) | Fold Change | Conc (ng/mL) | Fold Change | Conc (ng/mL) | Fold Change | Conc (ng/mL) | Fold Change | |
| 8e | 475.20 ± 4.27 | 7.23 | 1.29 | 7.58 | 12.89 | 13.86 | 0.548 | 11.90 |
| 8f | 365.60 ± 3.20 | 5.57 | ND | ND | ND | ND | ND | ND |
| 8g | 489.20 ± 4.13 | 7.54 | 1.52 | 8.94 | 13.98 | 15.03 | 0.587 | 12.76 |
| Doxorubicin | 503.20 ± 4.22 | 7.66 | 1.75 | 10.07 | 16.23 | 17.40 | 0.604 | 13.13 |
| Control | 65.64 | 1 | 0.17 | 1 | 0.93 | 1 | 0.046 | 1 |
| Compound No. | Bax | Bcl-2 | ||
|---|---|---|---|---|
| Conc (pg/mL) | Fold Change | Conc (ng/mL) | Fold Reduction | |
| 8e | 247.65 | 29.98 | 1.278 | 3.97 |
| 8g | 264.90 | 32.07 | 1.085 | 4.68 |
| Doxorubicin | 276.19 | 33.42 | 0.983 | 5.17 |
| Cont. | 8.26 | 1 | 5.086 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheref, E.M.; Elbastawesy, M.A.I.; Brown, A.B.; Shawky, A.M.; Gomaa, H.A.M.; Bräse, S.; Youssif, B.G.M. Design and Synthesis of (2-oxo-1,2-Dihydroquinolin-4-yl)-1,2,3-triazole Derivatives via Click Reaction: Potential Apoptotic Antiproliferative Agents. Molecules 2021, 26, 6798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226798
El-Sheref EM, Elbastawesy MAI, Brown AB, Shawky AM, Gomaa HAM, Bräse S, Youssif BGM. Design and Synthesis of (2-oxo-1,2-Dihydroquinolin-4-yl)-1,2,3-triazole Derivatives via Click Reaction: Potential Apoptotic Antiproliferative Agents. Molecules. 2021; 26(22):6798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226798
Chicago/Turabian StyleEl-Sheref, Essmat M., Mohammed A. I. Elbastawesy, Alan B. Brown, Ahmed M. Shawky, Hesham A. M. Gomaa, Stefan Bräse, and Bahaa G. M. Youssif. 2021. "Design and Synthesis of (2-oxo-1,2-Dihydroquinolin-4-yl)-1,2,3-triazole Derivatives via Click Reaction: Potential Apoptotic Antiproliferative Agents" Molecules 26, no. 22: 6798. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226798