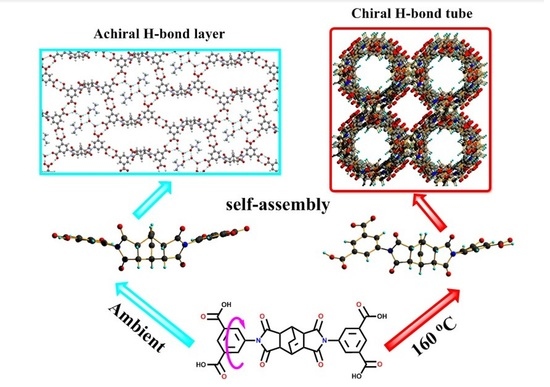
Two Unexpected Temperature-Induced Supermolecular Isomers from Multi-Topic Carboxylic Acid: Hydrogen Bonding Layer or Helix Tube
Abstract
:1. Introduction
2. Experimental Section
2.1. General Materials and Methods
2.1.1. Preparation of [(H4PTTA)(H2O)2(DMF)] (1-L)
2.1.2. Preparation of [(H4PTTA)(H2O)3]∙·Guest (1-H)
2.1.3. X-ray Crystallographic Analysis
3. Results and Discussion
3.1. Structural Description of [(H4PTTA)(H2O)2(DMF)] (1-L)
3.2. Structural Description of of [(H4PTTA)(H2O)3]∙·Guest (1-H)
3.3. Syntheses and Structural Comparison
3.4. CD Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pepinsky, R. Crystal engineering: New concept in crystallography. Phys. Rev. 1955, 100, 971. [Google Scholar]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, the Netherlands, 1989. [Google Scholar]
- Desiraju, G.R. Crystal Engineering: A Holistic View. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Brammer, L. Developments in inorganic crystal engineering. Chem. Soc. Rev. 2004, 33, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Braga, D.; Brammer, L.; Champness, N.R. New trends in crystal engineering. CrystEngComm 2005, 7, 1–19. [Google Scholar] [CrossRef]
- Hosseini, M.W. Molecular tectonics: From simple tectons to complex molecular networks. Acc. Chem. Res. 2005, 38, 313–323. [Google Scholar] [CrossRef]
- Sasaki, T.; Sakamoto, S.; Takamizawa, S. Organoferroelastic Crystal Prepared by Supramolecular Synthesis. Cryst. Growth Des. 2020, 20, 1935–1939. [Google Scholar] [CrossRef]
- Lin, R.B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional Porous Hydrogen-Bonded Organic Framework Materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef]
- Lehn, J.-M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 2007, 36, 151–160. [Google Scholar] [CrossRef]
- Bis, J.A.; McLaughlin, O.L.; Vishweshwar, P.; Zaworotko, M.J. Supramolecular heterocatemers and their role in cocrystal design. Cryst. Growth Des. 2006, 6, 2648–2650. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A. “Total synthesis” supramolecular style: Design and hydrogen-bond-directed assembly of ternary supermolecules. Angew. Chem. Int. Ed. 2001, 40, 3240–3242. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Friscic, T.; MacGillivray, L.R. Enforced face-to-face stacking of organic semiconductor building blocks within hydrogen-bonded molecular cocrystals. J. Am. Chem. Soc. 2006, 128, 2806–2807. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering-a new organic synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Joanna, A.B.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of supramolecular synthons: Persistent hydroxyl···pyridine hydrogen bonds in cocrystals that contain a cyano acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar]
- Rajput, L.; Biradha, K. Design of cocrystals via new and robust supramolecular synthon between carboxylic acid and secondary amide: Honeycomb network with jailed aromatics. Cryst. Growth Des. 2009, 9, 40–42. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.Y.; Xue, R.F.; Lu, X.F.; Chen, R.X.; Tao, X.T. Cocrystallization of N-donor type compounds with 5-sulfosalicylic acid: The effect of hydrogen-bonding supramolecular architectures. Sci. China Chem. 2012, 55, 138–144. [Google Scholar] [CrossRef]
- Kanters, J.A.; Roelofsen, G. Hydrogen-Bond Motifs of Carboxylic Acids: The α-Form of Monochloroacetic Acid. Acta Cryst. B 1976, 32, 3328–3331. [Google Scholar] [CrossRef] [Green Version]
- Kanters, J.A.; Roelofsen, G.; Feenstra, T. Hydrogen-Bond Motifs of Carboxylic Acids: The β-Form of Monochloroacetic Acid. Acta Cryst. B 1976, 32, 3331–3333. [Google Scholar] [CrossRef]
- Leiserowitz, L. Molecular Packing Modes. Carboxylic Acids. Acta Cryst. B 1976, 32, 775–802. [Google Scholar] [CrossRef] [Green Version]
- Rajput, L.; Jana, N.; Biradha, K. Carboxylic Acid and Phenolic Hydroxyl Interactions in the Crystal Structures of Co-Crystals/Clathrates of Trimesic Acid and Pyromellitic Acid with Phenolic Derivatives. Cryst. Growth Des. 2010, 10, 4565–4570. [Google Scholar] [CrossRef]
- D’Ascenzo, L.; Auffinger, P. A comprehensive classification and nomenclature of carboxyl–carboxyl(ate) supramolecular motifs and related catemers: Implications for biomolecular systems. Acta Cryst. B 2015, 71, 164–175. [Google Scholar] [CrossRef]
- Ou, G.; Wang, Q.; Zhou, Q.; Wang, X.-F. Phenol Derivatives as Co-Crystallized Templates to Modulate Trimesic-Acid-Based Hydrogen-Bonded Organic Molecular Frameworks. Crystals 2021, 11, 409. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhang, Y.-B.; Xue, W.; Qi, X.-L.; Chen, X.-M. Two temperature-induced isomers of metal-carboxylate frameworks based on different linear trinuclear Co3(RCOO)8 clusters exhibiting different magnetic behaviours. CrystEngComm 2010, 12, 3834–3839. [Google Scholar] [CrossRef]
- Du, L.-Y.; Shi, W.-J.; Hou, L.; Wang, Y.-Y.; Shi, Q.-Z.; Zhu, Z. Solvent or Temperature Induced Diverse Coordination Polymers of Silver(I) Sulfate and Bipyrazole Systems: Syntheses, Crystal Structures, Luminescence, and Sorption Properties. Inorg. Chem. 2013, 52, 14018–14027. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saha, B.K. Guest-induced isomerization of net and polymorphism in trimesic acid-arylamine complexes. Cryst. Growth Des. 2011, 11, 2194–2204. [Google Scholar] [CrossRef]
- Wang, X.-F.; Chen, Y.; Song, L.-P.; Fang, Z.; Zhang, J.; Shi, F.; Lin, Y.-W.; Sun, Y.; Zhang, Y.-B.; Rocha, J. Cooperative Capture of Uranyl Ions by a Carbonyl-Bearing Hierarchical-Porous Cu–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 18808–18812. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L.J. Single-crystal structure validation with the program PLATON. Appl. Crystallogr. 2003, 36, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Q.; Miao, X.; Qin, C.; Chu, D.; Cao, L. Adaptive Chirality of an Achiral Cucurbit[8]uril-Based Supramolecular Organic Framework for Chirality Induction in Water. Angew. Chem. Int. Ed. 2021, 60, 6744–6751. [Google Scholar] [CrossRef]
- Frank, F.C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Song, L.-P.; Zhou, J.; Zeng, Q.; Xie, R.; Wang, X.-F. Two chiral cadmium carboxylate framework isomers generated by spontaneous resolution: Synthesis, structures and properties. J. Coord. Chem. 2019, 72, 251–261. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, K.; Tian, J.; Liu, C.; Tian, J.; Jiang, F.; Yuan, D.; Zhang, J.; Chen, Q.; Hong, M. Induction of Chirality in a Metal–Organic Framework Built from Achiral Precursors. Angew. Chem. Int. Ed. 2021, 60, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Roszak, K.; Katrusiak, A. High-pressure and temperature dependence of the spontaneous resolution of 1,1′-binaphthyl enantiomers. Phys. Chem. Chem. Phys. 2018, 20, 5305–5311. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, R.; Táborosi, A.; Zucchi, C.; Pályi, G. Autosolvation: Architecture and Selection of Chiral Conformers in Alkylcobalt Carbonyl Molecular Clocks. Symmetry 2014, 6, 551–565. [Google Scholar] [CrossRef] [Green Version]







| 1-L | 1-H | |
|---|---|---|
| Empirical formula | C31H29N3O15 | C28H24N2O15 |
| Formula weight | 683.59 | 628.49 |
| Temperature | 293(2) | 296(2) |
| Crystal system | Triclinic | Tetragonal |
| Space group | P-1 | P4322 |
| Unit cell dimensions | ||
| a (Å) | 7.0188(2) | 11.0795(2) |
| b (Å) | 13.4051(7) | 11.0795(2) |
| c (Å) | 17.4948(8) | 35.0924(9) |
| α/° β/° γ/° | 101.590(4) 98.090(3) 94.526(3) | 90 90 90 |
| Volume/Å3 | 1586.66(12) | 4307.78(19) |
| Z | 2 | 4 |
| Dcalc./g·cm−3 | 1.431 | 0.969 |
| μ(MoKα)/mm−1 | 0.994 | 0.080 |
| F(000) | 712 | 1304 |
| Rint | 0.019 | 0.080 |
| Reflections collected | 9130 | 9844 |
| Observed reflections [I > 2σ(I)] | 4322 | 4446 |
| Flack parameter | 0 | 0.1(16) |
| Goodness-of-fit on F2 | 1.05 | 1.12 |
| R1 [I > 2σ(I)] | 0.0457 | 0.0461 |
| wR2 (all data) | 0.1226 | 0.1365 |
| Largest diff. peak and hole/e.Å−3 | 1.08 and −0.47 | 0.29 and −0.28 |
| 1-L | ||||
|---|---|---|---|---|
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
| O1W-H1WA···O6 #1 | 1.010 | 1.730 | 2.735(3) | 172 |
| O1W-H1WB···O2W | 1.060 | 1.640 | 2.684(3) | 167 |
| O3-H3···O4 #2 | 0.970 | 1.640 | 2.607(2) | 178 |
| O2W-H2WA···O13 #3 | 0.890 | 1.880 | 2.768(4) | 171 |
| O2W-H2WB···O13 #4 | 1.000 | 1.820 | 2.819(3) | 173 |
| O5-H5···O9 #5 | 0.940 | 1.710 | 2.628(2) | 166 |
| O10-H10···O1W #3 | 0.990 | 1.520 | 2.506(3) | 173 |
| O12-H12···O11 #6 | 1.050 | 1.560 | 2.593(2) | 168 |
| C6-H6···O8 #1 | 0.980 | 2.580 | 3.410(3) | 143 |
| C8-H8···O2 #7 | 0.930 | 2.540 | 3.309(3) | 140 |
| C31-H31B···O2 #7 | 0.960 | 2.510 | 3.366(4) | 148 |
| Symmetry codes: #1 = 2 − x, 2 − y, 1 − z; #2 = 1 − x, 2 − y, − z; #3 = − x, 1 − y, 1 − z; #4 = 1 + x, y, 1 + z; #5 = 2 + x, 1 + y, z; #6 = 1 − x, − y, 1 − z; #7 = − 1 + x, y, z. | ||||
| 1-H | ||||
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
| O2W-H2WA···O1 #1 | 0.85 | 2.11 | 2.883(2) | 150 |
| O2W-H2WB···O4 #2 | 0.85 | 1.95 | 2.784(3) | 167 |
| O2-H2A···O2 #3 | 0.86 | 1.6 | 2.440(2) | 164 |
| O3-H3···O2W | 0.81 | 1.94 | 2.713(3) | 160 |
| O1W-H1WA···O2W | 0.85 | 2.03 | 2.884(3) | 177 |
| C3-H3A···O3 #4 | 0.98 | 2.46 | 3.076(6) | 121 |
| Symmetry codes: #1 = x, 2 − y, 1/2 − z; #2 = − 1 + y, 1 + x, 1/4 − z; #3 = x, 1 − y, 1/2 − z; #4 = − 1 + y, x, 1/4 − z. | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Tan, C.; Zhou, J.; Lin, Y.-Y.; Wang, X.-F. Two Unexpected Temperature-Induced Supermolecular Isomers from Multi-Topic Carboxylic Acid: Hydrogen Bonding Layer or Helix Tube. Molecules 2021, 26, 6938. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226938
Li C, Tan C, Zhou J, Lin Y-Y, Wang X-F. Two Unexpected Temperature-Induced Supermolecular Isomers from Multi-Topic Carboxylic Acid: Hydrogen Bonding Layer or Helix Tube. Molecules. 2021; 26(22):6938. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226938
Chicago/Turabian StyleLi, Chunyang, Chunhong Tan, Juan Zhou, Yan-Yong Lin, and Xiao-Feng Wang. 2021. "Two Unexpected Temperature-Induced Supermolecular Isomers from Multi-Topic Carboxylic Acid: Hydrogen Bonding Layer or Helix Tube" Molecules 26, no. 22: 6938. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226938