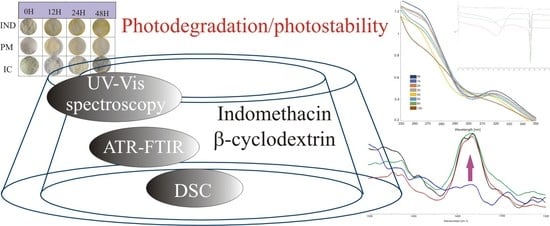
Preparation and Characterization of Indomethacin Supramolecular Systems with β-Cyclodextrin in Order to Estimate Photostability Improvement
Abstract
:1. Introduction
2. Results
2.1. Stoichiometry of IND-β-CD Inclusion Complex in Water
2.2. UV-Vis Studies of IND and Its Supramolecular Systems Photodegradation
2.3. DSC Analysis and Specific Heat-Capacity Data
2.4. Verification of the Photodegradation of Indomethacin Inclusion Complexes and Physical Mixtures with β-CD by FTIR Spectrophotometry and Attenuated Total Reflection Infrared Spectroscopy (ATR-FTIR)
3. Materials and Methods
3.1. Materials
3.2. Supramolecular Systems Preparation
3.3. Photostability Testing
3.4. Stoichiometry of Ind-β-CD Inclusion Complex in Liquid System
3.5. UV-Vis Studies
3.6. ATR-FTIR Spectroscopy Application for Verification of the Photodegradation of Indomethacin Inclusion Complexes and Physical Mixtures with β-CD
3.7. The Thermal Behaviour of the IND Supramolecular Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Janga, K.Y.; King, T.; Ji, N.; Sarabu, S.; Shadambikar, G.; Sawant, S.; Murthy, S.N. Photostability issues in pharma-ceutical dosage forms and photostabilization. Pharm. Sci. Tech. 2018, 19, 48–59. [Google Scholar] [CrossRef]
- Tønnesen, H.H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. 2001, 225, 1–14. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Harinarayana, D.; Madgulkar, A.R.; Pandya, S.J.; Jain, D.K. Improvement of photostability in for-mulation: A review. Asian J. Chem. 2008, 20, 5095–5108. [Google Scholar]
- Manconi, M.; Valenti, D.; Sinico, C.; Lai, F.; Loy, G.; Fadda, A.M. Niosomes as carriers for tretinoin: II. Influence of vesicular incorporation on tretinoin photostability. Int. J. Pharm. 2003, 260, 261–272. [Google Scholar] [CrossRef]
- Ragno, G.; Cione, E.; Garofalo, A.; Genchi, G.; Ioele, G.; Risoli, A. Design and monitoring of photostability systems for amlodipine dosage forms. Int. J. Pharm. 2003, 265, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Ioele, G.; De Luca, M.; Garofalo, A.; Ragno, G. Photosensitive drugs: A review on their photoprotection by lipo-somes and cyclodextrins. Drug Deliv. 2017, 24, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahabra, L.; Broadberry, G.; Le Gresley, A.; Najlah, M.; Khoder, M. Sunscreens containing cyclodextrin inclusion complexes for enhanced efficiency: A strategy for skin cancer prevention. Molecules 2021, 26, 1698. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Ioele, G.; Ragno, G. Review: 1,4-Dihydropyridine antihypertensive drugs: Recent advances in photo-stabilization strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalia, S.; Villani, S.; Scatturin, A.; Vandelli, M.A.; Forni, F. Complexation of the sunscreen agent, bu-tyl-methoxydibenzoylmethane, with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 1998, 175, 205–213. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; Neves de Lima, Á.A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Priotti, J.; Codina, A.V.; Vasconi, M.D.; Quiroga, A.D.; Hinrichsen, L.I.; Lamas, M.C. Synthesis and characterization of a new cyclodextrin derivative with improved properties to design oral dosage forms. Drug Deliv. Transl. Res. 2019, 9, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Diniz, T.C.; Pinto, T.C.C.; Menezes, P.D.P.; Silva, J.C.; Teles, R.B.D.A.; Ximenes, R.C.C.; Almeida, J.R.G.D.S. Cy-clodextrins improving the physicochemical and pharmacological properties of antidepressant drugs: A patent re-view. Expert Opin. Ther. Pat. 2018, 28, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J. Cyclodextrins as drug carriers in pharmaceutical tech-nology: The state of the art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Luckert, P. Treatment of chemically-induced intestinal cancers with indometacin. Proc. Soc. Exp. Biol. Med. 1981, 167, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, K.; Jiang, Z.; Chen, X.; Ma, J.; Ma, Q.; Duan, W. Indometacin inhibits the proliferation and activation of human pancreatic stellate cells through the downregulation of COX-2. Oncol. Rep. 2018, 39, 2243–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.L.; Zhang, G.Y.; Li, C.; Lin, J. Screening for novel protein targets of indomethacin in HCT116 human co-lon cancer cells using proteomics. Oncol. Lett. 2013, 6, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Weedon, A.C.; Wong, D.F. The photochemistry of indomethacin. J. Photochem. Photobiol. 1991, 61, 27–33. [Google Scholar] [CrossRef]
- Dabestani, R.; Sik, R.H.; Davis, D.G.; Dubay, G.; Chignell, C.F. Spectroscopic studies of cutaneous photosensitizing agents. Photochem. Photobiol. 1993, 58, 367–373. [Google Scholar] [CrossRef]
- Temussi, F.; Cermola, F.; DellaGreca, M.; Iesce, M.R.; Passananti, M.; Previtera, L.; Zarrelli, A. Determination of photostability and photodegradation products of indomethacin in aqueous media. J. Pharm. Biomed. Anal. 2011, 56, 678–683. [Google Scholar] [CrossRef]
- Skibiński, R. A study of photodegradation of quetiapine by the use of LC-MS/MS method. Open Chem. 2012, 10, 232–240. [Google Scholar] [CrossRef]
- Skibiński, R.; Komsta, Ł.; Inglot, T. Characterization of paliperidone photodegradation products by LC-Q-TOF multistage mass spectrometry. Biomed. Chromatogr. 2016, 30, 894–901. [Google Scholar] [CrossRef]
- Backensfeld, T.; Muller, B.W.; Wiese, M.; Seydel, J.K. Effect of cyclodextrin derivates on indomethacin stability in aqueos solution. Pharm. Res. 1990, 7, 484–490. [Google Scholar] [CrossRef]
- International Conference on Harminisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Guideline: Stability Testing Photostability Testing of New Drug Substances and Products, Q1B. 1996. Available online: https://database.ich.org/sites/default/files/Q1B%20Guideline.pdf (accessed on 1 September 2021).
- ICH Stability Testing: Photostability testing of new drug substances and products Q1B. Int. Conf. Harmon. 2003, 24. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 1 September 2021).
- Iram, F.; Iram, H.; Iqbal, A.; Husain, A. Forced degradation studies. J. Anal. Pharm. 2016, 3, 00073. [Google Scholar] [CrossRef] [Green Version]
- Jamrógiewicz, M. Consequences of new approach to chemical stability tests to active pharmaceutical ingredients. Front. Pharmacol. 2016, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brümmer, D. How to approach a forced degradation study. Life Sci. 2011, 31, 1–4. [Google Scholar]
- Alsante, K.M.; Ando, A.; Brown, R.; Ensing, J.; Hatajik, T.D.; Kong, W.; Tsuda, Y. The role of degradant profiling in active pharmaceutical ingredients and drug products. Drug Deliv. Rev. 2007, 59, 29–37. [Google Scholar] [CrossRef]
- Rawat, T.; Pandey, I.P. Forced degradation studies for drug substances and drug products- scientific and regula-tory considerations. J. Pharm. Sci Res. 2015, 7, 238–241. [Google Scholar]
- Jamrógiewicz, M.; Pieńkowska, K. Recent breakthroughs in the stability testing of pharmaceutical compounds. TrAC Trends Anal. Chem. 2019, 111, 118–127. [Google Scholar] [CrossRef]
- Nebsen, M.; Elzanfaly, E.S. Stability—indicating method and LC–MS-MS characterization of forced degradation products of sofosbuvir. J. Chromatogr. Sci. 2016, 54, 1631–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khovich, M.O.; Sharapova, A.; Blokhina, S.; Skachilova, S.; Shilova, E. A study of the inclusion complex of bioac-tive thiadiazole derivative with 2-hydroxypropyl-β-cyclodextrin: Preparation, characterization and physicochem-ical properties. J. Mol. Liq. 2019, 273, 653–662. [Google Scholar] [CrossRef]
- Misiuk, W. Investigation of inclusion complex of HP-γ-cyclodextrin with ceftazidime. J. Mol. Liq. 2016, 224, 387–392. [Google Scholar] [CrossRef]
- Wada-Hirai, A.; Shimizu, S.; Ichii, R.; Tsunoda, C.; Hiroshige, R.; Fujita, M.; Goto, S. Stabilization of the Metastable α–Form of Indomethacin Induced by the Addition of 2-Hydroxypropyl-β-Cyclodextrin, Causing Supersaturation (Spring) and Its Sustaining Deployment (Parachute). J. Pharm. Sci. 2021, 110, 3623–3630. [Google Scholar] [CrossRef]
- Nozawa, Y.; Morioka, Y.; Sadzuka, Y.; Miyagishima, A.; Hirota, S.; Guillory, J.K. Mechano-chemical formation of indomethacin β cyclodextrin inclusion compounds in powder phase roll mixtures. Pharm. Acta Helv. 1997, 72, 113–117. [Google Scholar] [CrossRef]
- Rudrangi, S.R.S.; Bhomia, R.; Trivedi, V.; Vine, G.J.; Mitchell, J.C.; Alexander, B.D.; Wicks, S.R. Influence of the preparation method on the physicochemical properties of indomethacin and methyl-β-cyclodextrin complexes. Int. J. Pharm. 2015, 479, 381–390. [Google Scholar] [CrossRef]
- Pessine, F.B.; Calderini, A.; Alexandrino, G.L. Cyclodextrin inclusion complexes probed by NMR techniques. In Magnetic Resonance Spectroscopy; InTechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Jamrógiewicz, M.; Wielgomas, B.; Strankowski, M. Evaluation of the photoprotective effect of β-cyclodextrin on the emission of volatile degradation products of ranitidine. J. Pharm. Biomed. Anal. 2014, 98, 113–119. [Google Scholar] [CrossRef]
- Jambhekar, S.; Casella, R.; Maher, T. The physicochemical characteristics and bioavailability of indomethacin from β-cyclodextrin, hydroxyethyl-β-cyclodextrin, and hydroxypropyl-β-cyclodextrin complexes. Int. J. Pharm. 2004, 270, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.H.; Wu, Y.M.; Xiong, X.L.; Zhang, Y. Inclusion complex of trimethoprim with β-cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 824–829. [Google Scholar] [CrossRef]
- Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany; New York, NY, USA, 1978. [Google Scholar]
- Kaneniwa, N.; Otsuka, M.; Hayashi, T. Physicochemical characterization of indomethacin polymorphs and the transformation kinetics in ethanol. Chem. Pharm. Bull. 1985, 33, 3447–3455. [Google Scholar] [CrossRef] [Green Version]
- Giordano, F.; Novak, C.; Moyano, J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Schneider, H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Rekharsky, M.V. Thermodynamics of hydrogen bond and hydrophobic interactions in cyclodextrin complexes. Biophys. J. 1996, 71, 2144–2154. [Google Scholar] [CrossRef] [Green Version]
- Benesi, H.A.; Hildebrand, J.H.A. The spectrophotometric investigation of the ineteraction of iodine with aromatic hydrocarbons. J. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Nigam, S.; Durocher, G. Inclusion complexes of some 3H-indoles with cyclodextrins studied through excited state dynamics and steady state absorption and fluorescence spectroscopy. J. Photochem. Photobiol. A 1997, 103, 143–152. [Google Scholar] [CrossRef]
- Baranowska, K.; Józefowicz, M. Spectroscopic studies of inclusion complexation between ortho derivatives of p-methylamino- benzoate and α- and γ-cyclodextrins. J. Mol. Liq. 2018, 265, 140–150. [Google Scholar] [CrossRef]
- Baranowska, K.; Mońka, M.; Kowalczyk, A.; Szpakowska, N.; Kaczyński, Z.; Bojarski, P.; Józefowicz, M. Spectro-scopic studies of the excited-state intramolecular proton and electron transfer processes of methyl benzoate deriv-atives in cucurbit[7]uril nanocage. J. Mol. Liq. 2020, 318, 113921. [Google Scholar] [CrossRef]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. 1928, 9, 113. [Google Scholar]
- European Pharmacopoeia 9.0, Indomethacin, 01/2017:0092. 2017. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 1 September 2021).
- Abu-Bakar, A.S.; Cran, M.; Moinuddin, K.A.M. Experimental investigation of effects of variation in heating rate, temperature and heat flux on fire properties of a non-charring polymer. J. Thermal Anal. Calorim. 2019, 137, 447–459. [Google Scholar] [CrossRef]












| Preparations | Pharmaceutical Form of the Preparation |
|---|---|
| Chrono-Indocin (Merck Sharp & Dohme-Chibret, France) | Capsules 75 mg |
| Indocid, (MSD, China) | Capsules 75 mg |
| Indocin (Iroko Pharms, USA) | Oral suspension 25 mg/5 mL–bottle 237 mL |
| Indocin (Lundbeck, USA) | Powder for injection solution (sodium salt)–vial 1 mg |
| Indomethacin (Avanthi, USA) | Prolonged release capsules 75 mg |
| Indomethacin (Fresenius Kabi, USA) | Lyophilized substance solution for injection–vial 1 mg |
| Indomethacin (G & W Labs, USA) | Suppositories 50 mg |
| Indomethacin (Ivax; Mylan; Sandoz, USA) | Capsules 25 mg and 50 mg |
| Indomet-ratiopharm (Ratiopharm, Denmark) | Hard capsules 25 mg and 50 mg Capsules retard 75 mg Suppositories 50 mg and 100 mg Gel 1%—50 mg and 100 mg |
| Metindol (Glaxosmithkline Pharmaceuticals, Poland) | Ointment 0.05 g/g|30 g |
| Elmetacin (Stada Arzneimittel AG, Germany) | Aerosol 0.01 g/g|50 mL |
| Sample | Molar Ratio IND:CD | Tm [°C, K] | Enthalpy [J/g] | Cp at 298 K [J⋅g−1⋅°C−1] | ΔCp at Tm |
|---|---|---|---|---|---|
| IND | - | 167.5; 440.5 | −101.19 | 1.08 | −6.74 |
| CD | - | 290–300; 563–573 | not tested | 1.44 | - |
| IC1 | 1:1 | 151.98; 424.98 160.99; 433.99 | −0.78; −1.31 | 1.51 | −2.52; −1.51 |
| IC2 | 1:0.5 | 151.62; 424.62 | −3.33 | 1.52 | 0.5 |
| IC3 | 1:0.1 | 160.75; 433.75 | −19.57 | 1.32 | −3.72 |
| PM1 | 1:1 | 161.76; 434.76 | −26.72 | 1.35 | 0.99 |
| PM2 | 1:0.5 | 161.13; 434.13 | −32.58 | 1.33 | 2.52 |
| PM3 | 1:0.1 | 162.65; 435.65 | −80.19 | 1.21 | 4.85 |
| Stoichiometry CD:API | β-CD | IND | Mass of Obtained Powder | |
|---|---|---|---|---|
| I. | 1:1 | 2.29 mmol (2.6 g) | 2.29 mmol (0.819 g) | 2.04 g |
| II. | 0.5:1 | 1.145 mmol (1.3 g) | 2.29 mmol (0.819 g) | 1.30 g |
| III. | 0.1:1 | 0.229 mmol (0.26 g) | 2.29 mmol (0.819 g) | 0.40 g |
| Components | [IND:CD] = [1:1] | [IND:CD] = [1:0.5] | [IND:CD] = [1:0.1] |
|---|---|---|---|
 | IC1 | IC2 | IC3 |
| + | |||
 | PM1 | PM2 | PM3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamrógiewicz, M.; Józefowicz, M. Preparation and Characterization of Indomethacin Supramolecular Systems with β-Cyclodextrin in Order to Estimate Photostability Improvement. Molecules 2021, 26, 7436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247436
Jamrógiewicz M, Józefowicz M. Preparation and Characterization of Indomethacin Supramolecular Systems with β-Cyclodextrin in Order to Estimate Photostability Improvement. Molecules. 2021; 26(24):7436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247436
Chicago/Turabian StyleJamrógiewicz, Marzena, and Marek Józefowicz. 2021. "Preparation and Characterization of Indomethacin Supramolecular Systems with β-Cyclodextrin in Order to Estimate Photostability Improvement" Molecules 26, no. 24: 7436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247436