Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Preparation of Mushroom Extracts
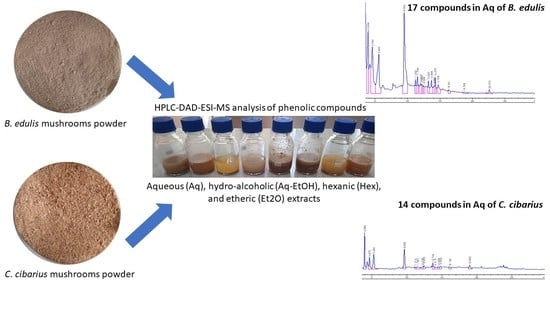
3.2.1. Raw Materials
3.2.2. Solvent Extraction
3.3. Determination of Mushroom Powders Proximate Composition
3.4. Determination of Total Phenolic Content
3.5. Determination of Total Flavonoid Content
3.6. Determination of Antioxidant Capacity by DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
3.7. Identification and Quantification of Polyphenolic Compounds by HPLC-DAD-ESI-MS
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wendiro, D.; Wacoo, A.P.; Wise, G. Identifying indigenous practices for cultivation of wild saprophytic mushrooms: Responding to the need for sustainable utilization of natural resources. J. Ethnobiol. Ethnomed. 2019, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Peintner, U.; Schwarz, S.; Mešić, A.; Moreau, P.-A.; Moreno, G.; Saviuc, P. Mycophilic or mycophobic? Legislation and guidelines on wild mushroom commerce reveal different consumption behaviour in European countries. PLoS ONE 2013, 8, e63926. [Google Scholar] [CrossRef] [Green Version]
- Kumari, D.; Reddy, M.S.; Upadhyay, R.C. Nutritional composition and antioxidant activities of 18 different wild Cantharellus mushrooms of Northwestern Himalayas. Food Sci. Technol. Int. 2015, 17, 557–567. [Google Scholar] [CrossRef]
- Gençcelep, H.; Uzun, Y.; Tunçtürk, Y.; Demirel, K. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009, 113, 1033–1036. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robaszkiewicz, A.; Bartosz, G.; Lawrynowicz, M.; Soszyński, M. The role of polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J. Nutr. Metab. 2010, 2010, 173274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. BioMed Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roleira, F.M.F.; Varela, C.L.; Costa, S.C.; Tavares-da-Silva, E.J. Phenolic derivatives from medicinal herbs and plant extracts: Anticancer effects and synthetic approaches to modulate biological activity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 115–156. [Google Scholar]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Yim, H.S.; Chye, F.Y.; Ho, S.K.; Ho, C.W. Phenolic profiles of selected edible wild mushrooms as affected by extraction solvent, time and temperature. Asian J. Food Agro-Ind. 2009, 2, 392–401. [Google Scholar]
- Alispahić, A.; Šapčanin, A.; Salihović, M.; Ramić, E.; Dedić, A.; Pazalja, M. Phenolic content and antioxidant activity of mushroom extracts from Bosnian market. Bull. Chem. Technol. Bosnia Herzeg. 2015, 44, 5–8. [Google Scholar]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Zavastin, D.E.; Bujor, A.; Tuchiluș, C.; Mircea, C.G.; Gherman, S.P.; Aprotosoaie, A.C.; Miron, A. Studies on antioxidant, antihyperglycemic and antimicrobial effects of edible mushrooms Boletus Edulis and Cantharellus cibarius. J. Plant Dev. 2016, 23, 87–95. [Google Scholar]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Keleş, A.; Koca, İ.; Gençcelep, H. Antioxidant properties of wild edible mushrooms. J. Food Process. Technol. 2011, 2, 130. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. sabel CFR Ferreira (2007) Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Yamac, M. Evaluation of the antioxidant activity of four edible mushrooms from the Central Anatolia, Eskisehir—Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresour. Technol. 2008, 99, 6651–6655. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Kuka, M.; Cakste, I. Bioactive Compounds in Latvian Wild Edible Mushroom Boletus edulis. In Proceedings of the FOODBALT-2011, 6th Baltic Conference on Food Science and Technology “Innovations for Food Science and Production”, Jelgava, Latvia, 5–6 May 2011; Straumite, E., Ed.; LLU: Jelgava, Latvia, 2011; pp. 116–120. [Google Scholar]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Petrović, J.; Papandreou, M.; Glamočlija, J.; Ćirić, A.; Baskakis, C.; Proestos, C.; Lamari, F.; Zoumpoulakis, P.; Soković, M. Different extraction methodologies and their influence on the bioactivity of the wild edible mushroom Laetiporus sulphureus (Bull.) Murrill. Food Funct. 2014, 5, 2948. [Google Scholar] [CrossRef]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT-Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Mishra, K.K.; Pal, R.S.; Bhatt, J.C. Comparison of antioxidant properties in cap and stipe of Lentinula edodes—A medicinal mushroom. Emir. J. Food Agric. 2015, 27, 562–569. [Google Scholar] [CrossRef] [Green Version]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudha, G.; Janardhanan, A.; Moorthy, A.; Chinnasamy, M.; Gunasekaran, S.; Thimmaraju, A.; Gopalan, J. Comparative study on the antioxidant activity of methanolic and aqueous extracts from the fruiting bodies of an edible mushroom Pleurotus djamor. Food Sci. Biotechnol. 2016, 25, 371–377. [Google Scholar] [CrossRef]
- Azieana, J.; Zainon, M.N.; Noriham, A.; Rohana, M.N. Total phenolic and flavonoid content and antioxidant activities of ten Malaysian wild mushrooms. Open Access Libr. J. 2017, 4, e3987. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Targeted metabolites analysis in wild Boletus species. LWT-Food Sci. Technol. 2011, 44, 1343–1348. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT-Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Çayan, F.; Tel-Çayan, G.; Deveci, E.; Duru, M.E. A comprehensive study on phenolic compounds and bioactive properties of five mushroom species via chemometric approach. J. Food Process. Preserv. 2021, 45, e15695. [Google Scholar] [CrossRef]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT-Food Sci. Technol. 2019, 110, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, B.; Kała, K.; Sułkowska-Ziaja, K.; Szewczyk, A.; Łojewski, M.; Rojowski, J. Analysis of the content of phenolic compounds in in vitro culture of some edible mushrooms (Basidiomycota). Med. Int. Rev. 2015, 26, 146–152. [Google Scholar]
- Miletić, N.; Popović, B.; Mitrović, O.; Kandić, M.; Leposavić, A. Phenolic compounds and antioxidant capacity of dried and candied fruits commonly consumed in Serbia. Czech J. Food Sci. 2014, 32, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, M.; Yang, H.; Zhou, H.; Yang, H. Production, physico-chemical characterization and antioxidant activity of natural melanin from submerged cultures of the mushroom Auricularia auricula. Food Biosci. 2018, 26, 49–56. [Google Scholar] [CrossRef]
- International Standard Organization. ISO 712:2009: Cereals and Cereal Products—Determination of Moisture Content—Reference Method; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Standard Organization. ISO 20483:2013: Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Teklit, G.A. Chemical composition and nutritional value of the most widely used mushrooms cultivated in Mekelle Tigray Ethiopia. J. Nutr. Food Sci. 2015, 5, 408. [Google Scholar] [CrossRef]
- Nagy, M.; Semeniuc, C.A.; Socaci, S.A.; Pop, C.R.; Rotar, A.M.; Sălăgean, C.D.; Tofană, M. Utilization of brewer’s spent grain and mushrooms in fortification of smoked sausages. Food Sci. Technol. Camp. 2017, 37, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomolecules 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Yang, F.; Wang, H.; Feng, G.; Zhang, S.; Wang, J.; Cui, L. Rapid identification of chemical constituents in Hericium erinaceus based on LC-MS/MS metabolomics. J. Food Qual. 2021, 40, 5560626. [Google Scholar] [CrossRef]

| Mushrooms Powder of: | Type of Extract | TPC | TFC | TEAC |
|---|---|---|---|---|
| B. edulis | Aq | 3.73 ± 0.007 a | 0.20 ±0.002 a | 1.90 ± 0.003 a |
| Aq-EtOH | 3.42 ± 0.003 b | 0.09 ± 0.005 b | 1.79 ± 0.014 b | |
| Hex | 0.48 ± 0.005 d | 0.05 ± 0.002 c | 0.23 ± 0.005 d | |
| Et2O | 0.79 ± 0.006 c | 0.05 ± 0.000 c | 0.35 ± 0.019 c | |
| C. cibarius | Aq | 0.79 ± 0.006 a | 0.03 ± 0.000 c | 1.12 ± 0.005 a |
| Aq-EtOH | 0.66 ± 0.006 b | 0.02 ± 0.000 d | 1.00 ± 0.003 b | |
| Hex | 0.58 ± 0.003 c | 0.28 ±0.005 b | 0.28 ± 0.009 d | |
| Et2O | 0.29 ± 0.005 d | 0.30 ± 0.000 a | 0.37 ± 0.005 c |
| Mushroom Species | Type of Sample | Type of Solvent Used | TPC | TFC | Antioxidant Activity by DPPH Assay | Reference |
|---|---|---|---|---|---|---|
| B. edulis | Lyophilized mushrooms | Pure methanol | 5.03 mg GAE/g | 1.75 mg CAE/g | - | [23] |
| Air-dried mushrooms | Pure methanol | 31.64 mg GAE/g extract | 0.458 mg QUE/g extract | 80.15% at an extract concentration of 1 mg/mL | [24] | |
| Freeze-dried mushrooms powder | Pure methanol | ≈5.5 mg GAE/g | ≈2.0 mg CAE/g | - | [9] | |
| Air-dried mushrooms powder (93.5% dry matter) | 80% Methanol | 12.78 mg GAE/g | - | 93.18% 3.95 mg/mL EC50 | [20] | |
| Freeze-dried mushrooms powder | Pure methanol | 8.0 mg GAE/g dw for B. edulis f. pinicola 7.3 mg GAE/g dw for B. edulis f. beticola | 0.13 mg QUE/g dw for B. edulis f. pinicola 0.13 mg QUE/g dw for B. edulis f. beticola | ≈79% for B. edulis f. pinicola ≈60% for B. edulis f. beticola | [26] | |
| Water | 12.5 mg GAE/g dw for B. edulis f. pinicola 11.2 mg GAE/g dw for B. edulis f. beticola | 0.37 mg QUE/g dw for B. edulis f. pinicola 0.33 mg QUE/g dw for B. edulis f. beticola | ≈81% for B. edulis f. pinicola ≈79% for B. edulis f. beticola | |||
| Lyophilized mushrooms powder | Acetone/water (4:1, v/v) | 28.56 mg GAE/g extract | - | 0.43 mg/mL EC50 | [34] | |
| Air dried mushrooms (at 25 °C for 15 days) | 70% Ethanol | 21.32 mg GAE/g extract | 116.64 mg QUE/g extract | 0.62 mg/mL IC50 | [10] | |
| 70% Methanol | 18.96 mg GAE/g extract | 97.5 mg QUE/g extract | 0.73 mg/mL IC50 | |||
| Hot water | 17.22 mg GAE/g extract | 82.85 mg QUE/g extract | 0.57 mg/mL IC50 | |||
| Cold water | 15.98 mg GAE/g extract | 61.42 mg QUE/g extract | 0.66 mg/mL IC50 | |||
| Dried mushrooms powder | 80% Ethanol | 35.56 mg GAE/g | - | 87.74% RSA | [16] | |
| Air-dried in the shade mushrooms powder | 96% Ethanol | 35.83 mg/g | - | 411.63 µg/mL EC50 | [18] | |
| Methanol/water (1:1, v/v) | 72.78 mg/g | - | 151.44 µg/mL EC50 | |||
| C. cibarius | Lyophilized mushrooms | Pure methanol | 0.88 mg GAE/g | 0.67 mg CAE/g | - | [23] |
| Dried mushrooms powder | Pure methanol | 1.75 mg GAE/g extract | - | 19.65 mg/mL EC50 | [25] | |
| Freeze-dried mushrooms powder | Pure methanol | ≈2.0 mg GAE/g | ~ 1.5 mg CAE/g | - | [9] | |
| Fresh mushrooms (6.5% dry matter) | Pure methanol | 11.94 mg GAE/g | 1.72 mg CAE/g | - | [3] | |
| Water | - | - | - | |||
| Lyophilized mushrooms powder | 2 M Hydrochloric acid solution | - | - | - | [38] | |
| Air-dried in the shade mushrooms powder | 96% Ethanol | 11.27 mg/g | - | >833.34 µg/mL EC50 | [18] | |
| Methanol/water (1:1, v/v) | 11.53 mg/g | - | 730.37 µg/mL EC50 | |||
| Dried mushrooms (98.2% dry matter) | Acetone/water/acetic acid (70:29.5:0.5, v/v/v) | 3.20 mg GAE/g | 0.35 mg CAE/g | 10.92 µmol TE/g | [35] | |
| Dried mushrooms powder (at 60 °C) | 60% Methanol | 1.41 mg GAE/g dw | 0.27 mg CAE/g dw | 64.10% RSA | [19] |
| Crt. No. | Compound | Chemical Class | Chemical Subclass | Aq | Aq-EtOH | Hex | Et2O |
|---|---|---|---|---|---|---|---|
| 1 | 2,4-Dihydroxybenzoic acid | PAs | HBAS | 590.70 ± 2.842 a | 177.96 ± 2.210 b | 0.62 ± 0.006 c | 5.27 ± 0.063 c |
| 2 | Gallic acid | PAs | HBAS | 371.46 ± 3.157 a | 235.06 ± 3.160 b | 0.75 ± 0.017 c | 2.08 ± 0.003 c |
| 3 | Syringic acid | PAs | HBAS | 934.19 ± 3.161 a | 578.51 ± 3.476 b | 0.35 ± 0.006 c | 8.65 ± 0.063 c |
| 4 | Protocatechuic acid 4-O-glucoside | PAs | HBAS | 1735.35 ± 3.789 a | 1603.44 ± 3.473 b | 7.92 ± 0.025 d | 18.95 ± 0.035 c |
| 5 | 1-O-Galloyl-β-D-glucose | PAs | HBAS | 82.88 ± 0.205 a | 42.65 ± 0.221 b | n.d. | n.d. |
| 6 | p-Hydroxybenzoic acid 4-O-glucoside | PAs | HBAS | 129.31 ± 0.339 b | 132.48 ± 0.316 a | n.d. | n.d. |
| 7 | Apigenin 7-O-glucoside | FVs | FVes | 82.05 ± 0.196 a | 60.16 ± 0.189 b | n.d. | n.d. |
| 8 | p-Hydroxybenzoic acid | PAs | HBAS | 51.62 ± 0.079 a | 49.55 ± 0.063 b | n.d. | n.d. |
| 9 | Catechin | FVs | FVals | 122.48 ± 0.319 a | 95.78 ± 0.297 b | n.d. | n.d. |
| 10 | Isorhamnetin-3-O-glucoside | FVs | FVols | 70.86 ± 0.196 a | 61.98 ± 0.189 b | n.d. | n.d. |
| 11 | Quercetin 3-O-acetyl-rhamnoside | FVs | FVols | 76.49 ± 0.194 a | 45.90 ± 0.221 b | n.d. | n.d. |
| 12 | Epicatechin | FVs | FVals | 74.07 ± 0.221 b | 95.32 ± 1.894 a | n.d. | n.d. |
| 13 | Myricetin 3-O-glucoside | FVs | FVols | 83.70 ± 0.189 a | 76.81 ± 0.221 b | n.d. | n.d. |
| 14 | Rutin | FVs | FVols | 41.34 ± 0.205 a | 30.98 ± 0.221 b | n.d. | n.d. |
| 15 | Quercetin 3-O-malonyl-glucoside | FVs | FVols | 25.10 ± 0.126 a | 23.27 ± 0.079 b | n.d. | n.d. |
| 16 | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | FVs | FVols | 6.12 ± 0.032 a | 5.87 ± 0.063 b | n.d. | n.d. |
| 17 | trans-Cinnamic acid | PAs | HCAS | 154.68 ± 0.316 a | 85.28 ± 0.191 b | 39.71 ± 0.158 d | 54.45 ± 0.065 c |
| Total content | 4632.38 | 3400.99 | 49.35 | 89.40 | |||
| Crt. No. | Compound | Chemical Class | Chemical Subclass | Aq | Aq-EtOH | Hex | Et2O |
|---|---|---|---|---|---|---|---|
| 1 | 2,4-Dihydroxybenzoic acid | PAs | HBAS | 202.48 ± 2.217 a | 90.33 ± 0.322 b | 0.69 ± 0.006 c | 1.217 ± 0.016 c |
| 2 | Gallic acid | PAs | HBAS | 112.51 ± 0.319 a | 63.37 ± 0.101 b | n.d. | 0.286 ±0.025 c |
| 3 | Syringic acid | PAs | HBAS | 166.69 ± 1.895 a | 171.60 ± 1.901 a | n.d. | 0.087 ± 0.006 b |
| 4 | Protocatechuic acid 4-O-glucoside | PAs | HBAS | 179.90 ± 1.932b | 253.54 ± 0.316 a | 5.40 ± 0.032 d | 20.67 ± 0.095 c |
| 5 | 1-O-Galloyl-β-D-glucose | PAs | HBAS | 14.43 ± 0.063 a | 7.72 ± 0.028 b | n.d. | n.d. |
| 6 | p-Hydroxybenzoic acid 4-O-glucoside | PAs | HBAS | 48.76 ± 0.095 a | 23.20 ± 0.095 b | n.d. | n.d. |
| 7 | p-Hydroxybenzoic acid | PAs | HBAS | 25.98 ± 0.126 b | 67.15 ± 0.221 a | n.d. | n.d. |
| 8 | Catechin | FVs | FVals | 111.94 ± 0.341 b | 115.46 ± 0.357 a | n.d. | n.d. |
| 9 | Quercetin 3-O-acetyl-rhamnoside | FVs | FVols | 12.75 ± 0.028 b | 18.14 ± 0.063 a | n.d. | n.d. |
| 10 | Epicatechin | FVs | FVals | 153.81 ± 0.253 b | 194.39 ± 2.210 b | n.d. | n.d. |
| 11 | Myricetin 3-O-glucoside | FVs | FVols | 12.00 ± 0.022 a | 6.12 ± 0.028 b | n.d. | n.d. |
| 12 | Rutin | FVs | FVols | 27.00 ± 0.189 a | 22.03 ± 0.025 b | n.d. | n.d. |
| 13 | Quercetin 3-O-malonyl-glucoside | FVs | FVols | 8.94 ± 0.022 a | 6.94 ± 0.021 b | n.d. | n.d. |
| 14 | Quercetin 3-O-glucosyl-rhamnosyl-glucoside | FVs | FVols | 11.51 ± 0.025 b | 22.03 ± 0.032 a | n.d. | n.d. |
| Total content | 1088.69 | 1062.02 | 6.09 | 22.26 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogarasi, M.; Socaciu, M.-I.; Sălăgean, C.-D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247508
Fogarasi M, Socaciu M-I, Sălăgean C-D, Ranga F, Fărcaș AC, Socaci SA, Socaciu C, Țibulcă D, Fogarasi S, Semeniuc CA. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules. 2021; 26(24):7508. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247508
Chicago/Turabian StyleFogarasi, Melinda, Maria-Ioana Socaciu, Claudiu-Dan Sălăgean, Floricuța Ranga, Anca Corina Fărcaș, Sonia Ancuța Socaci, Carmen Socaciu, Dorin Țibulcă, Szabolcs Fogarasi, and Cristina Anamaria Semeniuc. 2021. "Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania" Molecules 26, no. 24: 7508. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247508