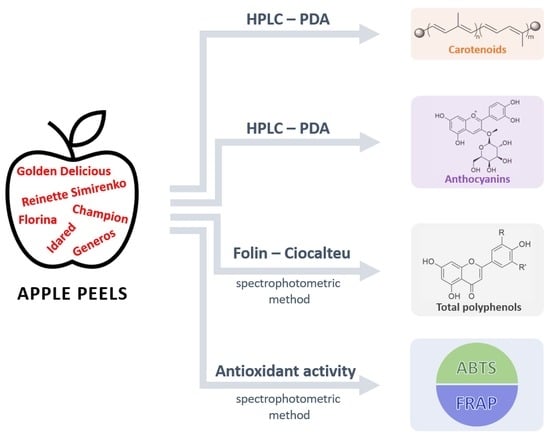
Phytochemical Content and Antioxidant Activity of Malus domestica Borkh Peel Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carotenoid Content of Apple Peels
2.2. Total Phenolics Content of Apple Peels
Anthocyanin Content of Apple Peels
2.3. Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Extraction of Carotenoids
HPLC-PDA Analysis of Carotenoids from Apple Peels
3.3. Determination of Total Phenolics
3.4. Extraction of Anthocyanins
HPLC-PDA Analysis of Anthocyanins
3.5. Determination of Antioxidant Activities
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wijewardane, R.M.N.A.; Guleria, S.P.S. Effect of pre-cooling, fruit coating and packaging on postharvest quality of apple. J. Food Sci. Technol. 2013, 50, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Tuszyński, T. Antioxidant activity of apples--an impact of maturity stage and fruit part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar]
- Karaman, S.; Tütem, E.; Başkan, K.S.; Apak, R. Comparison of antioxidant capacity and phenolic composition of peel and flesh of some apple varieties. J. Sci. Food Agric. 2013, 93, 867–875. [Google Scholar] [CrossRef]
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Ali Nawaz Ranjha, M.M.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T.; Sabtain, B.; et al. A Critical Review on Presence of Polyphenols in Commercial Varieties of Apple Peel, their Extraction and Health Benefits. Op. Acc. J. Bio. Sci. Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, N.; Rupasinghe, H.P. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, R.H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2008, 56, 9905–9910. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, V.P.; Girija, S.; Soumya, R.S.; Raghu, K.G. Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Funct. 2014, 5, 502–511. [Google Scholar] [CrossRef]
- Kheirvari, M.; Ardekani, M.K.; Anbara, T. Polyphenol-rich diet, an efficient strategy after bariatric surgery. Obes. Med. 2021, 26, 100365. [Google Scholar] [CrossRef]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Proper. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant. Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.E.; Dougall, D.K. Regulation of skin color in apples. Crit. Rev. Plant. Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Dar, J.A.; Wani, A.A.; Ahmed, M.; Nazir, R.; Zargar, S.M.; Javaid, K. Peel colour in apple (Malus × domestica Borkh.): An economic quality parameter in fruit market. Sci. Hortic. 2019, 244, 50–60. [Google Scholar] [CrossRef]
- Peng, T.; Moriguchi, T. The molecular network regulating the coloration in apple. Sci. Hortic. 2013, 163, 1–9. [Google Scholar] [CrossRef]
- Ju, Z.; Liu, C.; Yuan, Y.; Wang, Y.; Liu, G. Coloration potential, anthocyanin accumulation, and enzyme activity in fruit of commercial apple cultivars and their F1 progeny. Sci. Hortic. 1999, 79, 39–50. [Google Scholar] [CrossRef]
- Li, X.-J.; Hou, J.-H.; Zhang, G.-L.; Liu, R.-S.; Yang, Y.-G.; Hu, Y.-X.; Lin, J.-X. Comparison of anthocyanin accumulation and morpho-anatomical features in apple skin during color formation at two habitats. Sci. Hortic. 2004, 99, 41–53. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mo. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Molnár, P.; Deli, J.; Tanaka, T.; Kann, Y.; Tani, S.; Gyémánt, N.; Molnár, J.; Kawase, M. Carotenoids with anti-Helicobacter pylori activity from Golden delicious apple. Phytother. Res. 2010, 24, 644–648. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Schweiggert, R.M.; Vargas, E.; Conrad, J.; Hempel, J.; Gras, C.C.; Ziegler, J.U.; Mayer, A.; Jiménez, V.; Esquivel, P.; Carle, R. Carotenoids, carotenoid esters, and anthocyanins of yellow-, orange-, and red-peeled cashew apples (Anacardium occidentale L.). Food Chem. 2016, 200, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, D.A.; Schrader, L.E. Changes in pigment concentrations associated with sunburn browning of five apple cultivars. I. Chlorophylls and carotenoids. Plant Sci. 2009, 176, 78–83. [Google Scholar] [CrossRef]
- Muresan, E.A.; Muste, S.; Muresan, C.C.; Mudura, E.; Paucean, A.; Stan, L.; Romina Alina, V.; Cerbu, C.G.; Muresan, V. Assessment of polyphenols, chlorophylls, and carotenoids during developmental phases of three apple varieties. Rom. Biotechnol. Lett. 2017, 22, 12546–12553. [Google Scholar]
- Vondráková, Z.; Trávníčková, A.; Malbeck, J.; Haisel, D.; Černý, R.; Cvikrová, M. The effect of storage conditions on the carotenoid and phenolic acid contents of selected apple cultivars. Eur. Food Res. Technol. 2020, 246, 1783–1794. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Arcos, Y.; Godoy, F.; Flores-Ortiz, C.; Arenas, M.A.; Stange, C. Boosting carotenoid content in Malus domestica var. Fuji by expressing AtDXR through an Agrobacterium-mediated transformation method. Biotechnol. Bioeng. 2020, 117, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Sha, H.; Nie, J.; Wang, Y.; Yuan, Y.; Jia, D. An apple (Malus domestica) AP2/ERF transcription factor modulates carotenoid accumulation. Hortic. Res. 2021, 8, 223. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non-destructive assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Solovchenko, A.E.; Chivkunova, O.B. Patterns of pigment changes in apple fruits during adaptation to high sunlight and sunscald development. Plant Physiol. Biochem. 2002, 40, 679–684. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Avertcheva, O.V.; Merzlyak, M.N. Elevated sunlight promotes ripening-associated pigment changes in apple fruit. Postharvest Biol. Technol. 2006, 40, 183–189. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Gudkovsky, V.A. Relationships between chlorophyll and carotenoid pigments during on- and off-tree ripening of apple fruit as revealed non-destructively with reflectance spectroscopy. Postharvest Biol. Technol. 2005, 38, 9–17. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. The influence of exposure to light on the phenolic content of ‘Fuji’ apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Nagy, A.; Riczu, P.; Tamás, J. Spectral evaluation of apple fruit ripening and pigment content alteration. Sci. Hortic. 2016, 201, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Duda-Chodak, A.D.A.; Tarko, T.; Satora, P.; Sroka, P.; Tuszynski, T. The profile of polyphenols and antioxidant properties of selected apple cultivars grown in Poland. J. Fruit Ornam. Plant Res. 2010, 18, 39–50. [Google Scholar]
- Loncaric, A.; Matanovic, K.; Ferrer, P.; Kovac, T.; Sarkanj, B.; Skendrovic Babojelic, M.; Lores, M. Peel of Traditional Apple Varieties as a Great Source of Bioactive Compounds: Extraction by Micro-Matrix Solid-Phase Dispersion. Foods 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Massini, L.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Valorisation of Apple Peels. Eur. Food Res. Rev. 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Hermes, V.S.; Pagno, C.; Castagna, A.; Mannucci, A.; Sgherri, C.; Pinzino, C.; Ranieri, A.; Flôres, S.H.; Rios, A.d.O. Phenolic enrichment in apple skin following post-harvest fruit UV-B treatment. Postharvest Biol. Technol. 2018, 138, 37–45. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Kokalj, D.; Zlatić, E.; Cigić, B.; Kobav, M.B.; Vidrih, R. Postharvest flavonol and anthocyanin accumulation in three apple cultivars in response to blue-light-emitting diode light. Sci. Hortic. 2019, 257, 108711. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Chemical quality parameters and anthocyanin pattern of red-fleshed Weirouge apples. J. Appl. Bot. Food Qual. 2006, 80, 82–87. [Google Scholar]
- Awad, M.A.; de Jager, A. Formation of flavonoids, especially anthocyanin and chlorogenic acid in ‘Jonagold’ apple skin: Influences of growth regulators and fruit maturity. Sci. Hortic. 2002, 93, 257–266. [Google Scholar] [CrossRef]
- Wijewardane, R.M.N.A.; Guleria, S.P.S. Combined Effects of Pre-cooling, Application of Natural Extracts and Packaging on the Storage Quality of Apple (Malus domestica) cv. Royal Delicious. Trop. Agric. Res. 2009, 21, 10–20. [Google Scholar] [CrossRef]
- Kunradi-Vieira, F.G.; Da Silva Campelo Borges, G.; Copetti, C.; Da Valdemiro Gonzaga, L.; Costa Nunes, E.; Fett, R. Activity and contents of polyphenolic antioxidants in the whole fruit, flesh and peel of three apple cultivars. Arch. Latinoam. Nutr. 2009, 59, 101–106. [Google Scholar]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in Polyphenolics and Antioxidant Activity of Traditional Apple Cultivars from West Himalaya, Uttarakhand. Hortic. Plant. J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Schlatterer, J.; Breithaupt, D.E. Xanthophylls in commercial egg yolks: Quantification and identification by HPLC and LC-(APCI)MS using a C30 phase. J. Agric. Food Chem. 2006, 54, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Pintea, A.; Dugo, P.; Torre, G.; Pop, R.M.; Mondello, L. Determination of Carotenoids and their Esters in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochem. Anal. 2012, 23, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Meth Enzymol; Academic Press: Cambridge, MS, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Arnao, M.B.; Cano, A.; Alcolea, J.F.; Acosta, M. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem. Anal. 2001, 23, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Total Phenolic Content (mg GAE/kg FW) | Anthocyanins (mg/kg FW) | |||
|---|---|---|---|---|
| Cyanidin-3-O-galactoside | Cyanidin-3-O-glucoside | Cyanidin-3-O-arabinoside | ||
| Champion | 2143 ± 102 b | 292 ± 67 b | 18.08 ± 9 b | trace |
| Generos | 2056 ± 119 c | 175.32 ± 32 c | 11.45 ± 6.3 c | 23.22 ± 5.4 c |
| Idared | 2209 ± 132 b | 289.37 ± 54 b | 6.23 ± 0.97 d | 40.58 ± 7.2 a |
| Florina | 2723 ± 139 a | 396 ± 72 a | 29.74 ± 9.21 a | 35 ± 6.67 b |
| Golden Delicious | 1600 ± 99 d | nd | nd | nd |
| Reinette Simirenko | 1468 ± 89 e | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, M.; Bunea, A.; Ioan, C.R.; Ioan, B.C.; Socaci, S.; Viorel, M. Phytochemical Content and Antioxidant Activity of Malus domestica Borkh Peel Extracts. Molecules 2021, 26, 7636. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247636
Vasile M, Bunea A, Ioan CR, Ioan BC, Socaci S, Viorel M. Phytochemical Content and Antioxidant Activity of Malus domestica Borkh Peel Extracts. Molecules. 2021; 26(24):7636. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247636
Chicago/Turabian StyleVasile, Melnic, Andrea Bunea, Chira Romeo Ioan, Bunea Claudiu Ioan, Sonia Socaci, and Mitre Viorel. 2021. "Phytochemical Content and Antioxidant Activity of Malus domestica Borkh Peel Extracts" Molecules 26, no. 24: 7636. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26247636