Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer
Abstract
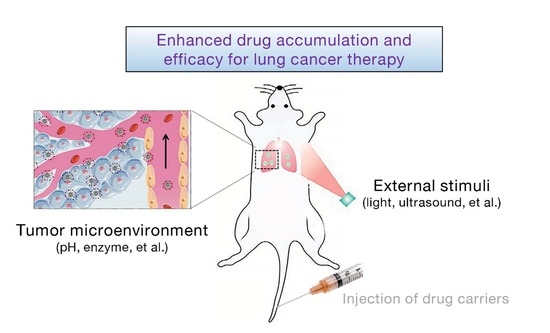
:1. Introduction
2. Light-Responsive Nanocarriers
3. Ultrasound-Responsive Nanocarriers
4. PH-Responsive Nanocarriers
5. Enzyme-Responsive Nanocarriers
6. Discussion and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.; Tammemagi, M.C.; Couraud, S.; Baldwin, D.R.; Borondy-Kitts, A.; Yankelevitz, D.; Lewis, J.; Grannis, F.; Kauczor, H.U.; von Stackelberg, O.; et al. Lung Screening Benefits and Challenges: A Review of The Data and Outline for Implementation. J. Thorac. Oncol. 2021, 16, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Horvath, L.; Thienpont, B.; Zhao, L.; Wolf, D.; Pircher, A. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC)—Novel approaches and future outlook. Mol. Cancer 2020, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Tumbrink, H.L.; Heimsoeth, A.; Sos, M.L. The next tier of EGFR resistance mutations in lung cancer. Oncogene 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Burgess, D.J. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm. Sin. B 2020, 10, 2110–2124. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Di, J.; Xie, F.; Xu, Y. When liposomes met antibodies: Drug delivery and beyond. Adv. Drug Deliv. Rev. 2020, 154, 151–162. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Lai, J.J. Three significant highlights of controlled drug delivery over the past 55 years: PEGylation, ADCs, and EPR. Adv. Drug Deliv. Rev. 2020, 158, 2–3. [Google Scholar] [CrossRef]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhi, Z.; Liu, D.; Wang, D.; Shao, Y.; Yan, K.; Meng, L.; Yu, D. Acid-responsive and biologically degradable polyphosphazene nanodrugs for efficient drug delivery. ACS Biomater. Sci. Eng. 2020, 6, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ji, S.; Guan, Y.; Mu, X.; Fang, S.; Lu, Y.; Zhou, X.; Sun, J.; Li, Z. Esterase-responsive polypeptide vesicles as fast-response and sustained-release nanocompartments for fibroblast-exempt drug delivery. Biomacromolecules 2020, 21, 5093–5103. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA hydrogel-based gene editing and drug delivery systems. Adv. Drug Deliv. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, J.; Huang, Z.; Chen, Y.; Pan, S.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Stimuli-responsive release and efficient siRNA delivery in non-small cell lung cancer by a poly(l-histidine)-based multifunctional nanoplatform. J. Mater. Chem. B 2020, 8, 1616–1628. [Google Scholar] [CrossRef]
- Feng, W.; Zong, M.; Wan, L.; Yu, X.; Yu, W. pH/redox sequentially responsive nanoparticles with size shrinkage properties achieve deep tumor penetration and reversal of multidrug resistance. Biomater. Sci. 2020, 8, 4767–4778. [Google Scholar] [CrossRef]
- Gai, C.; Liu, C.; Wu, X.; Yu, M.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020, 11, 751. [Google Scholar] [CrossRef]
- Akbaba, H.; Erel-Akbaba, G.; Kotmakci, M.; Baspinar, Y. Enhanced cellular uptake and gene silencing activity of survivin-siRNA via ultrasound-mediated nanobubbles in lung cancer cells. Pharm. Res. 2020, 37, 165. [Google Scholar] [CrossRef] [PubMed]
- Bomzon, Z.; Urman, N.; Wenger, C.; Giladi, M.; Weinberg, U.; Wasserman, Y.; Kirson, E.D.; Miranda, P.C.; Palti, Y. Modelling tumor treating fields for the treatment of lung-based tumors. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 6888–6891. [Google Scholar] [PubMed]
- Diaz, D.; Vidal, X.; Sunna, A.; Care, A. Bioengineering a light-responsive encapsulin nanoreactor: A potential tool for in vitro photodynamic therapy. ACS Appl. Mater. Interfaces 2021, 13, 7977–7986. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hong, Y.; Li, Y.; Hu, C.; Yip, T.C.; Yu, W.K.; Zhu, Y.; Fong, C.C.; Wang, W.; Au, S.K.; et al. Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics 2020, 10, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qin, Y.; Lee, J.; Liao, H.; Wang, N.; Davis, T.P.; Qiao, R.; Ling, D. Stimuli-responsive nano-assemblies for remotely controlled drug delivery. J. Control. Release 2020, 322, 566–592. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-F.; Lo, Y.-L.; Soorni, Y.; Su, C.-H.; Sivasoorian, S.S.; Yang, J.-Y.; Wang, L.-F. Near-infrared light-triggered drug release from ultraviolet- and redox-responsive polymersome encapsulated with core–shell upconversion nanoparticles for cancer therapy. ACS Appl. Bio. Mater. 2021, 4, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Park, J.Y.; Oh, Y.K. A microbial siderophore-inspired self-gelling hydrogel for noninvasive anticancer phototherapy. Cancer Res. 2019, 79, 6178–6189. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Lim, K.; Kim, S.S.; Thien, L.X.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Near infrared light-responsive heat-emitting hemoglobin hydrogels for photothermal cancer therapy. Colloids Surf. B Biointerfaces 2019, 176, 156–166. [Google Scholar] [CrossRef]
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhang, H.; Huang, X.Q.; Wan, H.Y.; Li, J.; Fan, X.X.; Luo, K.Q.; Wang, J.; Zhu, X.M.; Wang, J. Antiangiogenesis-combined photothermal therapy in the second near-infrared window at laser powers below the skin tolerance threshold. Nanomicro Lett. 2019, 11, 93. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Cai, S.; Mei, H.; He, Y.; Huang, D.; Shi, W.; Li, S.; Cao, J.; He, B. Photo-induced specific intracellular release EGFR inhibitor from enzyme/ROS-dual sensitive nano-platforms for molecular targeted-photodynamic combinational therapy of non-small cell lung cancer. J. Mater. Chem. B 2020, 8, 7931–7940. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Shi, X.; Zhou, J.; Zhou, L.; Wei, S. Use of an NIR-light-responsive CO nanodonor to improve the EPR effect in photothermal cancer treatment. Chem. Commun. 2018, 54, 13403–13406. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Qi, J.; Zhu, M.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Yu, H.; Sun, M.; et al. NIR-Triggered Sequentially Responsive Nanocarriers Amplified Cascade Synergistic Effect of Chemo-Photodynamic Therapy with Inspired Antitumor Immunity. ACS Appl. Mater. Interfaces 2020, 12, 32372–32387. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Xu, C.; Pu, K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 2021, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv. Mater. 2019, 31, e1902960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second near-infrared photothermal semiconducting polymer nanoadjuvant for enhanced cancer immunotherapy. Adv. Mater. 2021, 33, e2003458. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yan, J.; Long, S.; Xiong, H.; Wen, N.; Cai, S.; Wang, Y.; Peng, D.; Liu, Z.; Liu, Y. Tumor microenvironment and NIR laser dual-responsive release of berberine 9-O-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2020, 8, 4046–4055. [Google Scholar] [CrossRef]
- Xing, Y.; Zhou, Y.; Zhang, Y.; Zhang, C.; Deng, X.; Dong, C.; Shuang, S. Facile fabrication route of janus gold-mesoporous silica nanocarriers with dual-drug delivery for tumor therapy. ACS Biomater. Sci. Eng. 2020, 6, 1573–1581. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Deng, H.; Zheng, Z. Cinobufagin-loaded and folic acid-modified polydopamine nanomedicine combined with photothermal therapy for the treatment of lung cancer. Front. Chem. 2021, 9, 637754. [Google Scholar] [CrossRef]
- Gou, S.; Yang, J.; Ma, Y.; Zhang, X.; Zu, M.; Kang, T.; Liu, S.; Ke, B.; Xiao, B. Multi-responsive nanococktails with programmable targeting capacity for imaging-guided mitochondrial phototherapy combined with chemotherapy. J. Control. Release 2020, 327, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Zheng, J.; Li, B.; Zhang, D.; Jia, L. Redox/NIR dual-responsive MoS2 for synergetic chemo-photothermal therapy of cancer. J. Nanobiotechnol. 2019, 17, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Fan, Z.; Zuo, W.; Chen, Y.; Hou, Z.; Zhu, X. Self-distinguishing and stimulus-responsive carrier-free theranostic nanoagents for imaging-guided chemo-photothermal therapy in small-cell lung cancer. ACS Appl. Mater. Interfaces 2020, 12, 51314–51328. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Jakaria, M.G.; Meenach, S.A.; Bothun, G.D. Radiofrequency and near-infrared responsive core–shell nanostructures using layersome templates for cancer treatment. ACS Appl. Bio. Mater. 2020, 3, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tan, G.; Jiang, Y.; Yu, Z.; Ren, F. Rational design of multi-stimuli-responsive gold nanorod-curcumin conjugates for chemo-photothermal synergistic cancer therapy. Biomater. Sci. 2018, 6, 2905–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Sun, S.; Wang, P.; Ma, X.; Hou, R.; Liang, X. Anti-tumor metastasis via platelet inhibitor combined with photothermal therapy under activatable fluorescence/magnetic resonance bimodal imaging guidance. ACS Appl. Mater. Interfaces 2021, 13, 19679–19694. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cheng, R.; Zhao, C.; Sun, N.; Luo, H.; Chen, Y.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. Thermo- and pH-dual responsive polymeric micelles with upper critical solution temperature behavior for photoacoustic imaging-guided synergistic chemo-photothermal therapy against subcutaneous and metastatic breast tumors. Theranostics 2018, 8, 4097–4115. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Liu, W.; Zheng, Y.; Li, X.; Ye, J.; Li, B.; Chen, H.; Gao, Y. Near-infrared/pH dual-responsive nanocomplexes for targeted imaging and chemo/gene/photothermal tri-therapies of non-small cell lung cancer. Acta Biomater. 2020, 107, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-responsive materials for drug/gene delivery. Front. Pharmacol. 2019, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Entzian, K.; Aigner, A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; Ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-responsive nanocarriers in cancer treatment: A review. ACS Pharmacol. Transl. Sci. 2021, 4, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Erel-Akbaba, G.; Kotmakci, M.; Akbaba, H. Development and characterization of nanobubbles containing paclitaxel and survivin inhibitor YM155 against lung cancer. Int. J. Pharm. 2019, 566, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, L.; Guo, K.; Jia, Y.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 2019, 9, 5261–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khan, A.R.; Yang, X.; Shi, Y.; Zhao, X.; Zhai, G. A sonosensitiser-based polymeric nanoplatform for chemo-sonodynamic combination therapy of lung cancer. J. Nanobiotechnol. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yang, R.; Ren, J.; Liu, J.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Catalytically active CoFe2O4 nanoflowers for augmented sonodynamic and chemodynamic combination therapy with elicitation of robust immune response. ACS Nano 2021, 15, 11953–11969. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yao, M.; Shi, J.; Li, X.; Gao, Y.; Luo, Q.; Hou, R.; Liang, X.; Wang, F. High intensity focused ultrasound-responsive and ultrastable cerasomal perfluorocarbon nanodroplets for alleviating tumor multidrug resistance and epithelial-mesenchymal transition. ACS Nano 2020, 14, 15904–15918. [Google Scholar] [CrossRef]
- Lee, J.Y.; Crake, C.; Teo, B.; Carugo, D.; de Saint Victor, M.; Seth, A.; Stride, E. Ultrasound-enhanced siRNA delivery using magnetic nanoparticle-loaded chitosan-deoxycholic acid nanodroplets. Adv. Healthc. Mater. 2017, 6, 1601246. [Google Scholar] [CrossRef] [Green Version]
- Hamarat Sanlier, S.; Ak, G.; Yilmaz, H.; Unal, A.; Bozkaya, U.F.; Taniyan, G.; Yildirim, Y.; Yildiz Turkyilmaz, G. Development of ultrasound-triggered and magnetic-targeted nanobubble system for dual-drug delivery. J. Pharm. Sci. 2019, 108, 1272–1283. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core-shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnol. 2021, 19, 188. [Google Scholar] [CrossRef]
- Park, Y.I.; Kwon, S.H.; Lee, G.; Motoyama, K.; Kim, M.W.; Lin, M.; Niidome, T.; Choi, J.H.; Lee, R. pH-sensitive multi-drug liposomes targeting folate receptor beta for efficient treatment of non-small cell lung cancer. J. Control Release 2021, 330, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef] [PubMed]
- Shih, F.Y.; Jiang, W.P.; Lin, X.; Kuo, S.C.; Huang, G.J.; Hou, Y.C.; Chang, C.S.; Liu, Y.; Chiang, Y.T. A Novel pH-tunable secondary conformation containing mixed micellar system in anticancer treatment. Cancers 2020, 12, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Y.; Xiong, X.; Ming, Y.; Zhao, J.; Guo, X.; Yang, G.; Zhou, S. A Multifunctional micellar nanoplatform with pH-triggered cell penetration and nuclear targeting for effective cancer therapy and inhibition to lung metastasis. Adv. Healthc. Mater. 2018, 7, e1700974. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, X.; Zhang, J.; Pan, S.; Yang, C.; Wei, Y.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. pH-responsive hybrid nanoparticle with enhanced dissociation characteristic for siRNA delivery. Int. J. Nanomed. 2018, 13, 6885–6902. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ru, Y.; Gao, Y.; Li, J.; Mao, S. Layer-by-layer nanoparticles co-loading gemcitabine and platinum (IV) prodrugs for synergistic combination therapy of lung cancer. Drug Des. Devel. Ther. 2017, 11, 2631–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Li, W.; Yu, D. Atrial natriuretic peptide modified oleate adenosine prodrug lipid nanocarriers for the treatment of myocardial infarction: In vitro and in vivo evaluation. Drug Des. Devel. Ther. 2018, 12, 1697–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Che, S.; Hui, B.; Yang, Y.; Wang, X.; Zhang, X.; Qiang, Y.; Ma, H. Lung cancer therapy using doxorubicin and curcumin combination: Targeted prodrug based, pH sensitive nanomedicine. Biomed. Pharmacother. 2019, 112, 108614. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Zhang, C.; Zhi, X.; Cheng, J.; de la Fuente, J.M.; Song, J.; Cui, D. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018, 68, 308–319. [Google Scholar] [CrossRef]
- Chen, H.; Jin, Y.; Wang, J.; Wang, Y.; Jiang, W.; Dai, H.; Pang, S.; Lei, L.; Ji, J.; Wang, B. Design of smart targeted and responsive drug delivery systems with enhanced antibacterial properties. Nanoscale 2018, 10, 20946–20962. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, E.J.; Shi, H.; Lee, J.Y.; Chung, B.G.; Kim, J.S. Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomaterials 2018, 155, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Wang, Y.; Liu, X.; Hu, F.; Sun, J.; Yuan, H. Lipase-triggered water-responsive “Pandora’s Box” for cancer therapy: Toward induced neighboring effect and enhanced drug penetration. Adv. Mater. 2018, 30, e1706407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, M.; Toh, T.B.; Abdullah, N.L.B.; Chow, E.K. Stimuli-responsive nanodiamond-based biosensor for enhanced metastatic tumor site detection. SLAS Technol. 2018, 23, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 61–74. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, S.; Huo, M.; Chaudhuri, A.S.; Zhao, M.; Wu, Z.; Qi, X. Spatial controlled multistage nanocarriers through hybridization of dendrimers and gelatin nanoparticles for deep penetration and therapy into tumor tissue. Nanomedicine 2017, 13, 1399–1410. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.Y.; Guo, W.W.; Guo, N.N.; Chen, J.; Liu, H.N.; Xie, Z.Q.; Lin, M.T.; Wei, Q.C.; Gao, J.Q. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef]
- Sidi, L.; Luyang, C.; Kai, H.; Ning, C.; Qi, Z.; Kaikai, Y.; Hongzhao, Q.; Chaoyong, L.; Yanli, T.; Xin, H.; et al. Extracellular Delivery: Tumor microenvironment-tailored weakly cell-interacted extracellular delivery platform enables precise antibody release and function. Adv. Funct. Mater. 2019, 29, 1970301. [Google Scholar]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112027. [Google Scholar] [CrossRef]
- Ren, Q.; Liang, Z.; Jiang, X.; Gong, P.; Zhou, L.; Sun, Z.; Xiang, J.; Xu, Z.; Peng, X.; Li, S.; et al. Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy. Int. J. Biol. Macromol. 2019, 130, 845–852. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hong, E.H.; Jeong, J.Y.; Cho, J.; Seo, J.H.; Ko, H.J.; Cho, H.J. Esterase-sensitive cleavable histone deacetylase inhibitor-coupled hyaluronic acid nanoparticles for boosting anticancer activities against lung adenocarcinoma. Biomater. Sci. 2019, 7, 4624–4635. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yang, F.; Hu, J.; Li, T.; Wang, P.; Li, X.; Zhang, X. Rational designed highly sensitive NQO1-activated near-infrared fluorescent probe combined with NQO1 substrates in vivo: An innovative strategy for NQO1-overexpressing cancer theranostics. Eur. J. Med. Chem. 2021, 224, 113707. [Google Scholar] [CrossRef] [PubMed]
- Pradubyat, N.; Sakunrangsit, N.; Mutirangura, A.; Ketchart, W. NADPH: Quinone oxidoreductase 1 (NQO1) mediated anti-cancer effects of plumbagin in endocrine resistant MCF7 breast cancer cells. Phytomedicine 2020, 66, 153133. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jo, S.; Lee, Y.M.; Saravanakumar, G.; Lee, J.; Park, D.; Kim, W.J. Enzyme-triggered disassembly of polymeric micelles by controlled depolymerization via cascade cyclization for anticancer drug delivery. ACS Appl. Mater. Interfaces 2021, 13, 8060–8070. [Google Scholar] [CrossRef]
- Borys, N.; Dewhirst, M.W. Drug development of lyso-thermosensitive liposomal doxorubicin: Combining hyperthermia and thermosensitive drug delivery. Adv. Drug Deliv. Rev. 2021, 178, 113985. [Google Scholar] [CrossRef]
- Nardecchia, S.; Sánchez-Moreno, P.; Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical trials of thermosensitive nanomaterials: An overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Zahednezhad, F.; Zakeri-Milani, P.; Shahbazi Mojarrad, J.; Valizadeh, H. The latest advances of cisplatin liposomal formulations: Essentials for preparation and analysis. Expert Opin. Drug Deliv. 2020, 17, 523–541. [Google Scholar] [CrossRef]




| Stimuli | Specific Conditions | Nanocarriers | Diagnostic /Imaging | Therapeutics | Reference |
|---|---|---|---|---|---|
| Light | Near-infrared (NIR) light | Gold nanocage@manganese dioxide (AuNC@MnO2) nanoparticles | √ | √ | Lee et al., 2019 |
| Titania-coated gold nanobipyramids | √ | Chen et al., 2019 | |||
| Poly(l-lysine)-conjugated chlorin e6 (Ce6) derivative nanoparticle | √ | Zhang et al., 2020 | |||
| Palladium nanosheet (PdNS) | √ | Wang et al., 2018 | |||
| Semiconducting polymer nanoadjuvant (SPNIIIR) | √ | Li et al., 2021 | |||
| CE7Q/CQ/S | √ | √ | Li et al., 2020 | ||
| Short-wavelength and NIR light | O-nitrobenzyl ester modified polymersome with up-conversion nanoparticles | √ | Tsai et al., 2021 | ||
| Ultrasound | Mechanical effect | Perfluoropentane containing nanobubbles | √ | Baspinar et al., 2019 | |
| Chemical effect | PEGylated Co2Fe2O4 nanoflowers (CFP) | √ | √ | Fu et al., 2021 | |
| Synergistically therapeutic modality | Cerasomal perfluorocarbon nanodroplet (D-vPCs-O2) | √ | √ | Ma et al., 2020 | |
| EXO-DVDMS | √ | √ | Liu et al., 2019 | ||
| Liposome-based nanobubbles | √ | √ | Lee et al., 2017; | ||
| pH | pH 5.7–6.9 | CHEMS-based liposomes; HA-ERL/BEV-LPH nanoparticles; DOX-loaded mixed micelles; DA-TAT carrier; mPEG-PHis-PSD; U11-DOX/CUR nanoparticles; Cis-aconitic anhydride-modified doxorubicin | √ | Park et al., 2021; Pang et al., 2020; Shih et al., 2020; Jing et al., 2018; Shi et al., 2018; Hong et al., 2019; Xia et al., 2018 | |
| Enzyme | MMP-2 | Cur-P-NPs | √ | Han et al., 2017 | |
| MMP-9 | MMP-9-sensitive nanocarrier | √ | Sidi et al., 2019 | ||
| MMPs | A smart inhalable nanocarrier | √ | Vaghasiya et al., 2021 | ||
| HAase | HPGBCA | √ | Ren et al., 2019 | ||
| Esterase | Gold nanorod–curcumin conjugate, HAPBA | √ | Zhu et al., 2018; Lee et al., 2019 | ||
| NQO1 | QPA-P | √ | Park et al., 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Wu, J.; Liu, Y.; Lin, N.; Hu, J.; Zhang, B. Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer. Molecules 2022, 27, 948. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030948
Lin X, Wu J, Liu Y, Lin N, Hu J, Zhang B. Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer. Molecules. 2022; 27(3):948. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030948
Chicago/Turabian StyleLin, Xu, Jiahe Wu, Yupeng Liu, Nengming Lin, Jian Hu, and Bo Zhang. 2022. "Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer" Molecules 27, no. 3: 948. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030948