Resolution of a Configurationally Stable Hetero[4]helicene
Abstract
:1. Introduction
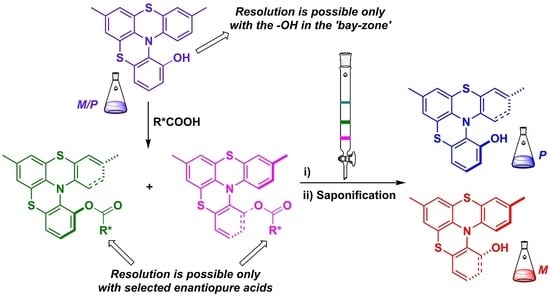
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. Chiroptical properties of symmetric double, triple, and multiple helicenes. Chem. Rev. 2021, 121, 2373–2412. [Google Scholar] [CrossRef] [PubMed]
- Reiné, P.; Ortuño, A.M.; Resa, S.; de Cienfuegos, L.Á.; Ribagorda, M.; Mota, A.J.; Abbate, S.; Longhi, G.; Miguel, D.; Cuerva, J.M. Enantiopure double ortho oligophenylethynylene-based helical structures with circularly polarized luminescence activity. ChemPhotoChem 2021, 6, e202100160. [Google Scholar] [CrossRef]
- Hong, J.; Xiao, X.; Liu, H.; Fu, L.; Wang, X.-C.; Zhou, L.; Wang, X.-Y.; Qiu, Z.; Cao, X.-Y.; Narita, A.; et al. X-shaped thiadiazole-containing double [7]heterohelicene with strong chiroptical response and π-stacked homochiral assembly. Chem. Com. 2021, 57, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, Z.; Huang, Z.; Cheng, R.; Yang, Y.; You, J. Triple Oxa[7]helicene with circularly polarized luminescence: Enhancing the dissymmetry factors via helicene subunit multiplication. Org. Lett. 2021, 23, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Borovkov, V. Helicene-based chiral auxiliaries and chirogenesis. Symmetry 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Neidle, S.; Waring, M. Molecular Aspects of Anticancer Drug-DNA Interactions; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- D’Incalci, M.; Sessa, C. DNA minor groove binding ligands: A new class of anticancer agents. Expert Opin. Invest. Drugs 1997, 6, 875–884. [Google Scholar] [CrossRef]
- Honzawa, S.; Okubo, H.; Anzai, S.; Yamaguchi, M.; Tsumoto, K.; Kumagai, I. Chiral recognition in the binding of helicenediamine to double strand DNA: Interactions between low molecular weight helical compounds and a helical polymer. Bioorg Med. Chem. 2002, 10, 3213–3218. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.X.; Sugiyama, H.; Umano, T.; Osuga, H.; Tanaka, K. (P)-helicene displays chiral selection in binding to Z-DNA. J. Am. Chem Soc. 2004, 126, 6566–6567. [Google Scholar] [CrossRef]
- Shinohara, K.; Sannohe, Y.; Kaieda, S.; Tanaka, K.; Osuga, H.; Tahara, H.; Xu, Y.; Kawase, T.; Bando, T.; Sugiyama, H. A chiral wedge molecule inhibits telomerase activity. J. Am. Chem. Soc. 2010, 132, 3778–3782. [Google Scholar] [CrossRef] [PubMed]
- Leydecker, T.; Wang, Z.M.; Torricelli, F.; Orgiu, E. Organic-based inverters: Basic concepts, materials, novel architectures and applications Chem. Soc. Rev. 2020, 49, 7627–7670. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Xie, X.; Ren, Y.; Zhang, B.; He, L.; Zhang, J.; Wang, L.-D.; Wang, P. A helicene-based molecular semiconductor enables 85 °C stable perovskite solar cells. ACS Energy Lett. 2021, 6, 1764–1772. [Google Scholar] [CrossRef]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef]
- Gingras, M.; Félix, G.; Peresutti, R. One hundred years of helicene chemistry. Part 2: Stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. [Google Scholar] [CrossRef]
- Lamanna, G.; Faggi, C.; Gasparrini, F.; Ciogli, A.; Villani, C.; Stephens, P.J.; Devlin, F.J.; Menichetti, S. Efficient thia-bridged triarylamine heterohelicenes: Synthesis, resolution, and absolute configuration determination. Chem. Eur. J. 2008, 14, 5747–5750. [Google Scholar] [CrossRef]
- Menichetti, S.; Cecchi, S.; Procacci, P.; Innocenti, M.; Becucci, L.; Franco, L.; Viglianisi, C. Thia-bridged triarylamine heterohelicene radical cations as redox-driven molecular switches. Chem. Commun. 2015, 51, 11452–11454. [Google Scholar] [CrossRef]
- Longhi, G.; Castiglioni, E.; Villani, C.; Sabia, R.; Menichetti, S.; Viglianisi, C.; Devlin, F.; Abbate, S. Chiroptical properties of the ground and excited states of two thia-bridged triarylamine heterohelicenes. J. Photochem. Photobiol. A Chem. 2016, 331, 138–145. [Google Scholar] [CrossRef]
- Menichetti, S.; Faggi, C.; Onori, M.; Piantini, S.; Ferreira, M.; Rocchi, S.; Lupi, M.; Marin, I.; Maggini, M.; Viglianisi, C. Thia-bridged triarylamine hetero[4]helicenes: Regioselective synthesis and functionalization C. Eur. J. Org. Chem. 2019, 2019, 168–175. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Baschieri, A.; Guo, Y.; Mollica, F.; Menichetti, S.; Lupi, M.; Viglianisi, C. SET and HAT/PCET acid-mediated oxidation processes in helical shaped fused bis-phenothiazines. ChemPhysChem 2021, 22, 1446–1454. [Google Scholar] [CrossRef]
- Lupi, M.; Menichetti, S.; Stagnaro, P.; Utzeri, R.; Viglianisi, C. Thia-bridged triarylamine[4]helicene-functionalized polynorbor nenes as redox-active pH-sensitive polymers. Synthesis 2021, 53, 2602–2611. [Google Scholar] [CrossRef]
- Gliemann, B.D.; Petrovic, A.G.; Zolnhofer, E.M.; Dral, P.O.; Hampel, F.; Breitenbruch, G.; Schulze, P.; Raghavan, V.; Meyer, K.; Polavarapu, P.L.; et al. Configurationally stable chiral dithia-bridged hetero[4]helicene radical cation: Electronic structure and absolute configuration. Chem. Asian J. 2017, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Giaconi, N.; Sorrentino, A.L.; Poggini, L.; Lupi, M.; Polewczyk, V.; Vinai, G.; Torelli, P.; Magnani, A.; Sessoli, R.; Menichetti, S.; et al. Stabilization of an enantiopure sub-monolayer of helicene radical cations on a Au(111) surface through noncovalent interactions. Angew. Chem. 2021, 133, 15404–15408. [Google Scholar] [CrossRef]
- Nuckolls, C.; Katz, T.J.; Katz, G.; Collings, P.J.; Castellanos, L. Synthesis and aggregation of a conjugated helical molecule. J. Am. Chem. Soc. 1999, 121, 79–88. [Google Scholar] [CrossRef]
- Nuckolls, C.; Katz, T.J.; Castellanos, L. Aggregation of conjugated helical molecules. J. Am. Chem. Soc. 1996, 118, 3767–3768. [Google Scholar] [CrossRef]
- Thongpanchang, T.; Paruch, K.; Katz, T.J.; Rheingold, A.L.; Lam, K.-C.; Liable-Sands, L. Why (1S)-camphanates are excellent resolving agents for helicen-1-ols and why they can be used to analyze absolute configurations. J. Org. Chem. 2000, 65, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Goldberg, N.M.; Katz, T.J. Efficient synthesis of functionalized [7] helicenes. J. Org. Chem. 1998, 63, 7456–7462. [Google Scholar] [CrossRef]
- Dreher, S.D.; Weix, D.J.; Katz, T.J. Easy synthesis of functionalized hetero [7] helicenes. J. Org. Chem. 1999, 64, 3671–3678. [Google Scholar] [CrossRef]
- Dreher, S.D.; Paruch, K.; Katz, T.J. Application of the Russig-Laatsch reaction to synthesize a bis [5] helicene chiral pocket for asymmetric catalysis. J. Org. Chem. 2000, 65, 815–822. [Google Scholar] [CrossRef]
- Dreher, S.D.; Katz, T.J.; Lam, K.-C.; Rheingold, A.L. First Friedel-Crafts diacylation of a phenanthrene as the basis for an efficient synthesis of nonracemic [7]helicenes. J. Org. Chem. 2000, 65, 7602–7608. [Google Scholar] [CrossRef]
- Li, H.-Y.; Nehira, T.; Hagiwara, M.; Harada, N. Total synthesis and absolute stereochemistry of the natural atropisomer of the biflavone 4′,4‴,7,7″-tetra-O-methylcupressuflavone. J. Org. Chem. 1997, 62, 7222–7227. [Google Scholar] [CrossRef] [PubMed]
- Fuji, K.; Sakurai, M.; Kinoshita, T.; Kawabata, T. Palladium-catalyzed asymmetric reduction of allylic esters with a new chiral monodentate ligand, 8-diphenylphosphino-8′-methoxy-1, 1′-binaphthyl. Tetrahedron Lett. 1998, 39, 6323–6326. [Google Scholar] [CrossRef]
- Ohmori, K.; Kitamura, M.; Suzuki, K. From axial chirality to central chiralities: Pinacol cyclization of 2,2′-biaryldicarbaldehyde to trans-9,10-dihydrophenanthrene-9,10-diol. Angew. Chem. Int. Ed. Engl. 1999, 38, 1226–1229. [Google Scholar] [CrossRef]
- Schaefer, T.; Penner, G.H. The conformational properties of some phenyl esters. Molecular orbital and nuclear magnetic resonance studies. Can. J. Chem. 1987, 65, 2175–2178. [Google Scholar] [CrossRef]
- Schaefer, T.; Sebastian, R.; Penner, G.H. Long-range formyl proton coupling constants of 4-X-phenyl formats (X=H, F, CH3, NO2) and 2,6-dichlorophenyl formate. Conformations in solution. Can. J. Chem. 1988, 66, 1787–1793. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Allinger, N.L.; Yuh, Y.H.; Lii, J.-H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 1989, 111, 8551–8566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01. Gaussian, Inc.: Wallingford, CT, USA, 2016. Available online: https://gaussian.com/citation/ (accessed on 28 December 2021).
- Zhao, Y.; Truhlar, D.G. Truhlar The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215. [Google Scholar]
- Coghill, A.M.; Garson, L.R. The ACS Style Guide, 3rd ed.; American Chemical Society: Washington, DC, USA, 2006; p. 274. [Google Scholar] [CrossRef]











| Chiral Auxiliary | Product | Yield | Resolution |
|---|---|---|---|
 7a (S)-(−)-Perillic acid | 8aaD1/8aaD2 | 60% | No resolution |
 7b Camphorsulfonyl chloride | 8abD1/8abD2 | 56% | No resolution |
 7c (−)-o,o’-Dibenzoyl-L-tartaric acid mono(dimethyl amide) | 8acD1/8acD2 | 56% | No resolution |
 7d (1S)-(+)-Ketopinic acid | 8adD1/8adD2 8bdD1/8bdD2 | 79% 55% | No resolution No resolution |
 7e (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid | 8aeD1/8aeD2 8beD1/8beD2 | 94% 58% | No resolution No resolution |
 7f (-)mono-(1R)-Menthylphthalate | 8afD1/8afD2 8bfD1/8bfD2 | 54% 45% | No resolution No resolution |
 7g (S)-N-Boc pipecolic acid | 8agD1/8agD2 8bgD1/8bgD2 | 69% 28%15% | No resolution Chromatographic resolution is possible |
 7h (1S)-(−)-Camphanic acid | 8ahD1/8ahD2 8bhD1/8bhD2 | 57% 37% 33% | No resolution Chromatographic resolution is possible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupi, M.; Onori, M.; Menichetti, S.; Abbate, S.; Longhi, G.; Viglianisi, C. Resolution of a Configurationally Stable Hetero[4]helicene. Molecules 2022, 27, 1160. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041160
Lupi M, Onori M, Menichetti S, Abbate S, Longhi G, Viglianisi C. Resolution of a Configurationally Stable Hetero[4]helicene. Molecules. 2022; 27(4):1160. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041160
Chicago/Turabian StyleLupi, Michela, Martina Onori, Stefano Menichetti, Sergio Abbate, Giovanna Longhi, and Caterina Viglianisi. 2022. "Resolution of a Configurationally Stable Hetero[4]helicene" Molecules 27, no. 4: 1160. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27041160