3D Printing of Monolithic Proteinaceous Cantilevers Using Regenerated Silk Fibroin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Highly Concentrated Silk Fibroin Solutions as Printing Inks
2.2. Characterization of Silk Fibroin Solutions
2.3. Representative 3D Prints with Silk Fibroin Inks
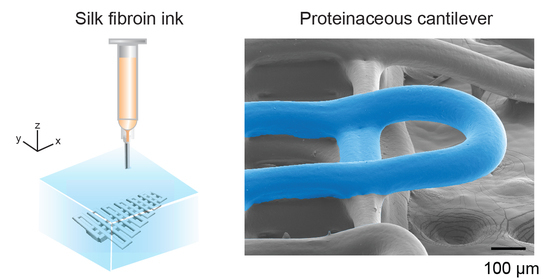
2.4. High Aspect Ratio 3D Prints and Cantilevers
3. Materials and Methods
3.1. Regeneration and Enrichment of Silk Fibroin Solution
3.2. Fluorescence Spectroscopy
3.3. UV-Vis Absorbance
3.4. Rheology
3.5. 3D Printing
3.6. Morphology
3.7. Tensile Tests
3.8. Computational Simulation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guzzi, E.A.; Tibbitt, M.W. Additive manufacturing of precision biomaterials. Adv. Mater. 2020, 32, 1901994. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Agostinacchio, F.; Ning, X.; Pei, Y.; Guo, C.; Khan, Y.; Cebe, P.; Motta, A.; Kaplan, D.L. Recent Advances in 3D printing with Protein-based Inks. Prog. Polym. Sci. 2021, 115, 101375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D extrusion bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2021, 34, 2103691. [Google Scholar] [CrossRef] [PubMed]
- Agostinacchio, F.; Mu, X.; Dirè, S.; Motta, A.; Kaplan, D.L. In situ 3D printing: Opportunities with silk inks. Trends Biotechnol. 2021, 39, 719–730. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M. Bioprinting: 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1970126. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Luo, Z.; Tang, G.; Mu, X.; Kuang, X.; Guo, J.; Zhao, Z.; Flores, R.S.; Jiang, Z. A multifunctional micropore-forming bioink with enhanced anti-bacterial and anti-inflammatory properties. Biofabrication 2022, 14, 024105. [Google Scholar] [CrossRef]
- Liao, J.; Ye, C.; Guo, J.; Garciamendez-Mijares, C.E.; Agrawal, P.; Kuang, X.; Japo, J.O.; Wang, Z.; Mu, X.; Li, W.; et al. 3D-printable colloidal photonic crystals. Mater. Today 2022. [Google Scholar] [CrossRef]
- Harrington, M.J.; Fratzl, P. Natural Load-Bearing Protein Materials. Prog. Mater. Sci. 2020, 120, 100767. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Włodarczyk-Biegun, M.K.; del Campo, A. 3D bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Latza, V.; Guerette, P.A.; Ding, D.; Amini, S.; Kumar, A.; Schmidt, I.; Keating, S.; Oxman, N.; Weaver, J.C.; Fratzl, P.; et al. Multi-scale thermal stability of a hard thermoplastic protein-based material. Nat. Commun. 2015, 6, 8313. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, C.; Vu, H.V.; Hanna, P.; Lechtig, A.; Qiu, Y.; Mu, X.; Ling, S.; Nazarian, A.; Lin, S.J. Thermoplastic moulding of regenerated silk. Nat. Mater. 2020, 19, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Parker, S.T.; Wang, X.; Kaplan, D.L.; Lewis, J.A. Direct-Write Assembly of Microperiodic Silk Fibroin Scaffolds for Tissue Engineering Applications. Adv. Funct. Mater. 2008, 18, 1883–1889. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef]
- Jose, R.R.; Brown, J.E.; Polido, K.E.; Omenetto, F.G.; Kaplan, D.L. Polyol-Silk bioink formulations as two-part room-temperature curable materials for 3D printing. ACS Biomater. Sci. Eng. 2015, 1, 780–788. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D bioprinting of self-standing silk-based bioink. Adv. Healthc. Mater. 2018, 7, 1701026. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 18016. [Google Scholar] [CrossRef]
- Rising, A.; Johansson, J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015, 11, 309. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Wang, Y.; Guo, C.; Li, Y.; Ling, S.; Huang, W.; Cebe, P.; Hsu, H.H.; De Ferrari, F.; Jiang, X.; et al. 3D Printing of Silk Protein Structures by Aqueous Solvent-Directed Molecular Assembly. Macromol. Biosci. 2020, 20, 1900191. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, J.; Ling, S. Bioinspired and biomimetic silk spinning. Compos. Commun. 2019, 13, 85–96. [Google Scholar] [CrossRef]
- Trucco, D.; Sharma, A.; Manferdini, C.; Gabusi, E.; Petretta, M.; Desando, G.; Ricotti, L.; Chakraborty, J.; Ghosh, S.; Lisignoli, G. Modeling and Fabrication of Silk Fibroin–Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater. Sci. Eng. 2021, 7, 3306–3320. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Midha, S.; Sharma, A.; Ghosh, S. Silk-based bioinks for 3D bioprinting. Adv. Healthc. Mater. 2018, 7, 1701204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D printing of silk fibroin for biomedical applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.M. Current and future trends of silk fibroin-based bioinks in 3D printing. J. 3D Printing Med. 2020, 4, 69–73. [Google Scholar] [CrossRef]
- Mu, X.; Fitzpatrick, V.; Kaplan, D.L. From Silk Spinning to 3D Printing: Polymer Manufacturing using Directed Hierarchical Molecular Assembly. Adv. Healthc. Mater. 2020, 9, 1901552. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Mu, X.; Kaplan, D.L. Engineering silk materials: From natural spinning to artificial processing. Appl. Phys. Rev. 2020, 7, 011313. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Johansson, J.; Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacht, K.; Jüngst, T.; Schweinlin, M.; Ewald, A.; Groll, J.; Scheibel, T. Biofabrication of cell-loaded 3D spider silk constructs. Angew. Chem. Int. Ed. 2015, 54, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Fast setting silk fibroin bioink for bioprinting of patient-specific memory-shape implants. Adv. Healthc. Mater. 2017, 6, 1701021. [Google Scholar] [CrossRef]
- Sun, L.; Parker, S.T.; Syoji, D.; Wang, X.; Lewis, J.A.; Kaplan, D.L. Direct-Write Assembly of 3D Silk/Hydroxyapatite Scaffolds for Bone Co-Cultures. Adv. Healthc. Mater. 2012, 1, 729–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hotz, B.; Ling, S.; Guo, J.; Haas, D.S.; Marelli, B.; Omenetto, F.; Lin, S.J.; Kaplan, D.L. Regenerated silk materials for functionalized silk orthopedic devices by mimicking natural processing. Biomaterials 2016, 110, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhang, L.; Peng, Q.; Sun, M.; Zhang, Y.; Shao, H.; Hu, X. Tough silk fibers prepared in air using a biomimetic microfluidic chip. Int. J. Biol. Macromol. 2014, 66, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of water content on the β-sheet formation, thermal stability, water removal, and mechanical properties of silk materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.; Cebe, P. Effect of water on the thermal properties of silk fibroin. Thermochim. Acta 2007, 461, 137–144. [Google Scholar] [CrossRef]
- Ishida, M.; Asakura, T.; Yokoi, M.; Saito, H. Solvent-And mechanical-treatment-induced conformational transition of silk fibroins studies by high-resolution solid-state carbon-13 NMR spectroscopy. Macromolecules 1990, 23, 88–94. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, G.; Knight, D.P.; Shao, Z.; Chen, X. Wet-Spinning of regenerated silk fiber from aqueous silk fibroin solution: Discussion of spinning parameters. Biomacromolecules 2009, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Cho, D.-W.; Lee, H. Development of Silk Fibroin Scaffolds by Using Indirect 3D-Bioprinting Technology. Micromachines 2022, 13, 43. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Chen, Y.; Bayat, A.; Yuan, X.-F. Rheology and electrospinning of regenerated Bombyx mori silk fibroin aqueous solutions. Biomacromolecules 2014, 15, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tarakanova, A.; Dinjaski, N.; Wang, Q.; Xia, X.; Chen, Y.; Wong, J.Y.; Buehler, M.J.; Kaplan, D.L. Design of Multistimuli Responsive Hydrogels Using Integrated Modeling and Genetically Engineered Silk–Elastin-Like Proteins. Adv. Funct. Mater. 2016, 26, 4113–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgakoudi, I.; Tsai, I.; Greiner, C.; Wong, C.; DeFelice, J.; Kaplan, D. Intrinsic fluorescence changes associated with the conformational state of silk fibroin in biomaterial matrices. Opt. Express 2007, 15, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Sahoo, J.K.; Cebe, P.; Kaplan, D.L. Photo-Crosslinked Silk Fibroin for 3D Printing. Polymers 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057. [Google Scholar] [CrossRef]
- Lim, B.T.; Kimura, T. Conformation-Associated anomalous tyrosine fluorescence of adrenodoxin. J. Biol. Chem. 1980, 255, 2440–2444. [Google Scholar] [CrossRef]
- Ross, J.A.; Laws, W.R.; Rousslang, K.W.; Wyssbrod, H.R. Tyrosine fluorescence and phosphorescence from proteins and polypeptides. In Topics in Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2002; pp. 1–64. [Google Scholar]
- Divya, O.; Mishra, A.K. Understanding the concept of concentration-dependent red-shift in synchronous fluorescence spectra: Prediction of λSFSmax and optimization of Δλ for synchronous fluorescence scan. Anal. Chim. Acta 2008, 630, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Bagheri, M.; Harden, J.L.; Kaplan, D.L. Tyrosine Templating in the Self-Assembly and Crystallization of Silk Fibroin. Biomacromolecules 2016, 17, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Es-Said, O.; Foyos, J.; Noorani, R.; Mendelson, M.; Marloth, R.; Pregger, B. Effect of layer orientation on mechanical properties of rapid prototyped samples. Mater. Manuf. Process. 2000, 15, 107–122. [Google Scholar] [CrossRef]
- Datta, P.; Vyas, V.; Dhara, S.; Chowdhury, A.R.; Barui, A. Anisotropy properties of tissues: A basis for fabrication of biomimetic anisotropic scaffolds for tissue engineering. J. Bionic Eng. 2019, 16, 842–868. [Google Scholar] [CrossRef]
- Smay, J.E.; Cesarano, J.; Lewis, J.A. Colloidal inks for directed assembly of 3-D periodic structures. Langmuir 2002, 18, 5429–5437. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971–1900978. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, C.; Kumarasena, A.; Omenetto, F.G.; Kaplan, D.L. 3D Printing of Functional Microalgal Silk Structures for Environmental Applications. ACS Biomater. Sci. Eng. 2019, 5, 4808–4816. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Dennis, P.B.; Tondiglia, V.P.; Nadeau, L.J.; Singh, K.M.; Drummy, L.F.; Partlow, B.P.; Brown, D.P.; Omenetto, F.G.; Kaplan, D.L. 3D Printing of Regenerated Silk Fibroin and Antibody-Containing Microstructures via Multiphoton Lithography. ACS Biomater. Sci. Eng. 2017, 3, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Li, Q.; Sun, S.-M.; Huang, J.-C.; Zheng, B.-Y.; Chen, Q.-D.; Shao, Z.-Z.; Sun, H.-B. Aqueous multiphoton lithography with multifunctional silk-centred bio-resists. Nat. Commun. 2015, 6, 8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, W.; Wang, Y.; Mu, X.; Ling, S.; Yu, H.; Chen, W.; Guo, C.; Watson, M.C.; Yu, Y. Stimuli-Responsive composite biopolymer actuators with selective spatial deformation behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 14602–14608. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Greenleaf, J.F.; Fatemi, M.; Insana, M. Selected methods for imaging elastic properties of biological tissues. Annu. Rev. Biomed. Eng. 2003, 5, 57–78. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, X.; Gonzalez-Obeso, C.; Xia, Z.; Sahoo, J.K.; Li, G.; Cebe, P.; Zhang, Y.S.; Kaplan, D.L. 3D Printing of Monolithic Proteinaceous Cantilevers Using Regenerated Silk Fibroin. Molecules 2022, 27, 2148. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072148
Mu X, Gonzalez-Obeso C, Xia Z, Sahoo JK, Li G, Cebe P, Zhang YS, Kaplan DL. 3D Printing of Monolithic Proteinaceous Cantilevers Using Regenerated Silk Fibroin. Molecules. 2022; 27(7):2148. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072148
Chicago/Turabian StyleMu, Xuan, Constancio Gonzalez-Obeso, Zhiyu Xia, Jugal Kishore Sahoo, Gang Li, Peggy Cebe, Yu Shrike Zhang, and David L. Kaplan. 2022. "3D Printing of Monolithic Proteinaceous Cantilevers Using Regenerated Silk Fibroin" Molecules 27, no. 7: 2148. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072148